1.1.9 Therapeutic Levels
Ernstmeyer & Christman - Open Resources for Nursing (Open RN)
Now that the concepts of dose-response, onset, peak, and duration have been discussed, it is important to understand the therapeutic window and therapeutic index.
Therapeutic Window
For every drug, there exists a dose that is minimally effective (the Effective Concentration) and another dose that is toxic (the Toxic Concentration). Between these doses is the therapeutic window, where the safest and most effective treatment will occur. For example, see Figure 1.9[1] for an illustration of the therapeutic window for warfarin, a medication used to prevent blood clotting. Too much warfarin administered causes bleeding and vitamin K is required as an antidote. Conversely, not enough warfarin administered for a client’s condition can cause clotting. Think of the therapeutic window (the green area on the graph) as the “perfect dose,” where clotting is prevented and yet bleeding does not occur.
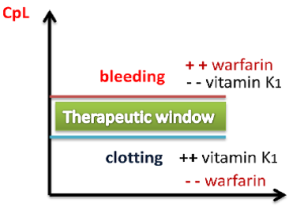
The effect of warfarin is monitored using a blood test called international normalized ration (INR). For clients receiving warfarin, nurses vigilantly monitor their INR levels to ensure the dosage appropriately reaches their therapeutic window and does not place them at risk for bleeding or clotting.
Peak and Trough Levels
Now let’s apply the idea of therapeutic window to the administration of medications requiring the monitoring of peak and trough levels, which is commonly required in the administration of specific IV antibiotics. The dosage of these medications is titrated, meaning adjusted for safety, to achieve a desired therapeutic effect for the client. Titration is accomplished by closely monitoring the peak and trough levels of the medication. A drug is said to be within the “therapeutic window” when the serum blood levels of an active drug remain consistently above the level of effective concentration (so that the medication is achieving its desired therapeutic effect) and consistently below the toxic level (so that no toxic effects are occurring).
A peak drug level is drawn after the medication is administered and is known to be at the highest level in the bloodstream. A trough level is drawn when the drug is at its lowest in the bloodstream, right before the next scheduled dose is given. Medications have a predicted reference range of normal values for peak and trough levels. These numbers assist the pharmacist and provider in gauging how the body is metabolizing, protein-binding, and excreting the drug and are used to adjust the prescribed dose to keep the medication within the therapeutic window. When administering IV medications that require peak or trough levels, it is vital for the nurse to plan the administration of the medication according to the timing of these blood draws.[2]
Therapeutic Index
The therapeutic index is a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of drug that produces a therapeutic effect versus the amount of drug that produces a toxic effect.
- A large (or high) therapeutic index number means there is a wide therapeutic window between the effective concentration and the toxic concentration of a medication, so the drug is relatively safe.
- A small (or low) therapeutic index number means there is a narrow therapeutic window between the effective concentration and the toxic concentration. A drug with a narrow therapeutic range (i.e., having little difference between toxic and therapeutic doses) often has the dosage titrated according to measurements of the actual blood levels achieved in the person taking it.
For example, phenytoin has a narrow therapeutic index between the effective and toxic concentrations. Clients who start taking phenytoin to control seizures have frequent peak and trough drug levels to ensure they achieve steady state with a therapeutic dose to prevent seizures without reaching toxic levels.
Critical Thinking Activity 1.10
Mr. Parker has been receiving gentamicin 80 mg IV three times daily to treat his infective endocarditis. He has his gentamicin level checked one hour after the end of his previous gentamicin infusion was completed. The result is 30 mcg/mL. Access the information below to determine the nurse’s course of action.
View information on therapeutic drug levels.
(After accessing the information, be sure to select “click to keep reading” in order to view drugs that are commonly checked, their target levels, and what abnormal results mean.)
Based on the results in the above client scenario, what action will the nurse take based on the result of the gentamicin level of 30 mcg/mL?
Note: Answers to the Critical Thinking activities can be found in the “Answer Key” section at the end of the book.
Next- 1.1.9.1 Evaluating Effects
Media Attributions
- Therapeutic Window © Shefaa Alasfoor is licensed under a CC BY-SA (Attribution ShareAlike) license
- ORN-Icons_internet-copy_internet-copy-300×300-1
- “Therapeutic Window” by Shefaa Alasfoor is licensed under CC BY-SA 3.0 ↵
- This work is a derivative of Principles of Pharmacology by LibreTexts and is licensed under CC BY-NC-SA 4.0 ↵
The hormone released by parathyroid glands and is involved in the regulation of blood calcium levels.
Learning Outcomes
By the end of this section, you should be able to:
- 10.2.1 Identify the characteristics of drugs used to treat myasthenia gravis.
- 10.2.2 Explain the indications, actions, adverse reactions, and interactions of drugs used to treat myasthenia gravis.
- 10.2.3 Describe nursing implications of drugs used to treat myasthenia gravis.
- 10.2.4 Explain the client education related to drugs used to treat myasthenia gravis.
Cholinergic agonists are also termed parasympathomimetics, and cholinergic antagonists (also known as anticholinergics) are termed parasympatholytics. (The anticholinergic agents will be discussed in Drugs to Treat Parkinson’s Disease and Multiple Sclerosis.) The PSNS has two general sites where drugs can act: (1) the synapses between preganglionic neurons and postganglionic neurons, and (2) the junctions between postganglionic neurons and their effector organs. The class of cholinergic agonists is subdivided into direct acting and indirect acting. The direct-acting cholinergic agonists interact with the postsynaptic cholinergic receptors and cause them to perform the same functions as if endogenous ACh was present. The indirect-acting cholinergic agonists are classified as AChE inhibitors and do not bind directly to receptors. AChE inhibitors prevent the enzyme from destroying acetylcholine. Because the enzyme is inhibited, there is more acetylcholine available for use. AChE inhibitors are subdivided into two categories: reversible and irreversible. These classes are discussed in the following sections.
Direct-Acting Cholinergic Agonists
Direct-acting cholinergic agonists mimic the effects of ACh when they bind to cholinergic receptors. They are considered direct acting because they have affinity (attracted to a receptor) and intrinsic activity (ability to stimulate a receptor) to these receptors. Direct-acting cholinergic agonists mainly bind to the muscarinic receptors of the PSNS. (Refer to Table 10.1 earlier in this chapter to see responses produced when muscarinic receptors are activated.) The most common therapeutic uses for these drugs are to promote urinary excretion and gastrointestinal (GI) motility/secretions. Additionally, there are several direct-acting cholinergic agonists that are found in ophthalmic formulations and induce miosis for the purpose of treating glaucoma because they help to relieve increased intraocular pressure. An example of a direct-acting cholinergic agonist in an ophthalmic formulation is pilocarpine. Pilocarpine also comes as an oral solution and is used to treat dry mouth associated with Sjogren’s syndrome or salivary gland damage. Due to the direct-acting cholinergic agonist’s selective action on muscarinic receptors, nicotinic responses are minimal or nonexistent; therefore, this class is not used in the treatment of MG.
Indirect-Acting Cholinergic Agonists: Reversible Acetylcholinesterase Inhibitors
Indirect-acting cholinergic agonists bind reversibly to AChE. They inhibit the enzyme that destroys acetylcholine, making acetylcholine more available. The specific mechanism of action is to delay the splitting of ACh into choline and acetone. Because AChE must degrade the drug in order to become unbound, less of this enzyme is available to break down ACh. This leaves more ACh to bind to the cholinergic receptors, intensifying its response at all the junctions where it serves as the neurotransmitter. The result of this action is prolonging access to ACh, allowing it to accumulate within the synaptic cleft; ACh continues binding to cholinergic receptors on the postsynaptic neuron, causing activation of that neuron. The effectiveness lasts until AChE is released. Once released, AChE will begin to break down ACh, and the effects of the drug will wear off. Reversible inhibitors produce effects of moderate duration. The optimal dose is determined by administering a small initial dose followed by gradual small increases until optimal muscle function is observed.
Pyridostigmine Bromide
Pyridostigmine bromide is the drug of choice for managing MG. This drug is formulated in a powder, syrup, or tablet. Tablets are available in immediate-release (IR) and extended-release (ER) forms. Both IR and ER forms may be needed to sustain effects. When adequate amounts of ACh are present at the NMJ, skeletal muscle is stimulated. The force of skeletal muscle contraction is increased at therapeutic doses. In contrast, too much drug can reduce the force of skeletal muscle strength because it leaves the NMJ in a state of constant depolarization.
Neostigmine
Neostigmine is administered only intravenously (IV). Essentially, it is used in the diagnosis and treatment of MG and reversal/recovery of the nondepolarizing neuromuscular blocking agents after surgery. The onset of action is 10–30 minutes, and peak effects occur in 20–30 minutes. Atropine should be readily available when infusing this drug.
A peripheral nerve stimulation device (also known as a train of four) is used to determine the degree of muscle relaxation based on muscle response after a stimulus is provided. This helps to determine if more medication is necessary when reversing any neuromuscular blockade postoperatively. The train of four count consists of four consecutive 2-Hz stimuli to a muscle group and the number of twitches evoked.
Table 10.2 lists common reversible AChE inhibitors and typical routes and dosing for adult clients.
| Drug | Routes and Dosage Ranges |
|---|---|
| Pyridostigmine bromide (Mestinon) |
Highly individualized. IR dosage range: 60–1500 mg orally daily; typical dose is 600 mg daily divided into 5 doses. ER dosage range: 180–540 mg orally daily or twice daily. |
| Neostigmine (Bloxiverz) |
Diagnosis of MG: 0.022 mg/kg intramuscularly. Reversal of nondepolarizing neuromuscular blocking agents: Dose is individualized to control symptoms: 0.03–0.07 mg/kg intravenously (IV). |
Adverse Effects and Contraindications
If the amount of pyridostigmine becomes too elevated, this can result in a cholinergic crisis. It is essential that the prescriber does not interpret skeletal muscle weakness as a sign of inadequate dosing because increasing the dose will elevate the risk of cholinergic crisis.
Safety Alert
Differentiating Between Cholinergic Crisis and Myasthenic Crisis
Taking too much of an AChE inhibitor can result in cholinergic crisis. Cholinergic and myasthenic crises share similar symptoms, such as muscle weakness or paralysis. The nurse must accurately distinguish between the two because the treatments are very different. A medication history or signs of excessive cholinergic stimulation (excess saliva, watery eyes, difficulty breathing, bradycardia, frequent urge to urinate, muscle twitching, and nausea/vomiting/diarrhea) can indicate a person is experiencing a cholinergic crisis not related to the myasthenia gravis. Stop direct or indirect cholinergic agonists until muscle strength improves.
Antidote for cholinergic crisis: Atropine (selective muscarinic antagonist) blocks the muscarinic receptors, which helps to reverse most of the signs/symptoms.
Antidote for myasthenic crisis: AChE inhibitor (pyridostigmine) will increase the necessary ACh levels.
(Sources: Adeyinka & Kondamudi, 2023; Health Union, 2023)
Apart from the adverse drug reactions associated with skeletal muscle, the majority of the adverse effects relate to the excessive stimulation of the muscarinic receptors. Intravenous atropine can alleviate the muscarinic effects. The fall in cardiac output can lead to hypotension. The diaphragm can be negatively affected, causing respiratory depression that is treated by mechanical ventilation with oxygen and not with medications. Pyridostigmine can cause bradycardia and exacerbate any underlying cardiac conduction abnormalities. Due to the constriction of the bronchi, asthmatics must be closely monitored when taking AChE inhibitors. Dysphonia (hoarseness) is the result of laryngospasms. Depending on the severity of the vomiting, diarrhea, diaphoresis, and urine output, dehydration can result.
Succinylcholine is a depolarizing neuromuscular blocker. Reversible AChE inhibitors decrease the breakdown of this drug; therefore, this combination can intensify the neuromuscular blockade. On the other hand, atropine can negate the effects of the AChE inhibitors.
Table 10.3 is a drug prototype table for indirect-acting reversible AChE inhibitors featuring pyridostigmine bromide. It lists drug class, mechanism of action, adult dosage, indications, therapeutic effects, drug and food interactions, adverse effects, and contraindications.
| Drug Class Reversible AChE inhibitor Mechanism of Action Causes reversible inhibition of AChE within the synapse, allowing more ACh to bind to cholinergic receptors and prolonging its effects |
Drug Dosage Highly individualized. IR dosage range: 60–1500 mg orally daily; typical dose is 600 mg daily divided into 5 doses. ER dosage range: 180–540 mg orally daily or twice daily. |
| Indications Management of MG symptoms Reversal of competitive (nondepolarizing) neuromuscular blockade Prevention/treatment of urinary retention in postoperative clients Therapeutic Effects Improves transmission of nerve impulses across the NMJ, increasing muscle strength and decreasing difficulty with chewing, swallowing, and speech, along with improvement or absence of ptosis Treats overdose from a competitive neuromuscular blocker |
Drug Interactions Succinylcholine Atropine Food Interactions No significant interactions |
| Adverse Effects Excess salivation and lacrimation Increased urination Diaphoresis Diarrhea Nausea/vomiting Bradycardia Decrease in cardiac output Bronchospasms Dysphagia Dysarthria Dysphonia Seizures Miosis Weakness, fasciculations, or paralysis of skeletal muscles |
Contraindications Mechanical obstruction of the intestine or urinary tract Caution: Bronchial asthma Cardiac conduction abnormalities, such as atrioventricular block |
Clinical Tip
Underdosing Versus Overdosing
Although failure of clients to show clinical improvement may reflect underdosage, it can also be indicative of overdosage. As is true of all cholinergic drugs, overdosage of pyridostigmine bromide may result in cholinergic crisis.
Nursing Implications
The nurse should do the following for clients who are taking an indirect-acting reversible AChE inhibitor:
- Monitor heart rate/rhythm and blood pressure periodically.
- Assess respiratory pattern (rate, depth, rhythm, effort) and airway patency.
- Time the administration of the drug so that peak effects occur at meals to help with eating and swallowing.
- Evaluate client’s ability to chew and swallow.
- Monitor client’s ease of ability to raise the eyelids (important sign of improvement).
- Monitor for clinical manifestations of dehydration if vomiting, diarrhea, or diaphoresis is present.
- Administer antiemetics to reduce nausea and vomiting.
- Provide client teaching regarding the drug and when to call the health care provider. See below for client teaching guidelines.
Client Teaching Guidelines
The client taking an indirect-acting reversible AChE inhibitor should:
- Understand that MG is not curable, so treatment is lifelong.
- Be able to recognize signs of therapeutic failure (ptosis, difficulty swallowing) and toxicity (excess muscarinic responses).
- Maintain records of the times the drug was administered, times at which fatigue occurred, and level of muscle strength before and after taking the drug.
- Wear a medic alert bracelet due to the potential of experiencing a crisis (myasthenic or cholinergic).
- Move and change positions slowly due to hypotension.
- Sit or lie down if dizziness occurs and wait until it subsides before standing or walking.
- Ensure adequate lighting to enhance vision due to the pupillary constriction.
- Ensure they have ready access to a restroom/commode due to the stimulatory effect on the GI and genitourinary (GU) systems.
- Contact their prescriber immediately if they have any difficulty with swallowing, speaking, hoarseness, or breathing.
- Space activities to obtain optimal benefit from the drug.
The client taking an indirect-acting reversible AChE inhibitor should not:
- Chew, break, or crush extended-release capsules.
- Rise or change positions quickly due to possible orthostatic hypotension.
- Stop drugs abruptly because symptoms will quickly reoccur.
- Overexert themselves; they should take rest periods between activities.
Indirect-Acting Cholinergic Agonists: Irreversible AChE Inhibitors
These drugs bind irreversibly to AChE. The effects of these drugs are prolonged—they will last until new molecules of cholinesterase are synthesized. Another method of reversing the inhibition of AChE, especially at the NMJ, is to administer pralidoxime, a cholinesterase reactivator. This drug is a specific antidote to poisoning by the irreversible cholinesterase inhibitor. The drug has no effect on reversible AChE inhibitors. This medication is administered either via IV or intramuscularly. Dosage is individualized according to the severity of symptoms.
The only therapeutic indication for this drug class is the treatment of glaucoma. For that indication, only one drug, echothiopate, is available. The drugs have limited use because they are highly toxic. Once they become absorbed, they will easily cross the blood–brain barrier, causing adverse effects.
Case Study
Read the following clinical scenario to answer the questions that follow.
Rae Lennon is a 38-year-old assistant professor at a small university. In November, they noticed that their vision was blurry, especially when grading papers. Rae said that they were seeing double. At first, they just assumed they were tired because their sleeping pattern had changed over the past 2 weeks and they were not getting enough sleep.
About a month after that, while drying their hair, Rae observed their left eyelid was drooping and it was becoming difficult to hold the hairdryer due to weakness in the arms. Over the Christmas break, Rae visited the primary care provider, who referred Rae to a local neurologist based on the symptoms. During Rae’s appointment with the neurologist, a thorough history and physical assessment were carried out. In addition, blood tests results revealed elevated levels of acetylcholine receptor antibodies. Based on these results, Rae was diagnosed with MG.
History
Overactive bladder
Current Medications
Oxybutynin 5 mg, 4 times daily
| Vital Signs | Physical Examination | |
|---|---|---|
| Temperature: | 98.2°F |
|
| Heart rate: | 72 beats/min | |
| Respiratory rate: | 18 breaths/min | |
| Blood pressure: | 112/64 mm Hg | |
| Oxygen saturation: | 96% on room air | |
| Height: | 5'6" | |
| Weight: | 138 lb | |
Trending Today
A New Medication for Myasthenia Gravis
The U.S. Food and Drug Administration (FDA) has approved a new medication, rozanolixizumab-noli (Rystiggo), for generalized MG in adults who are anti-acetylcholine receptor (AChR) or anti-MuSK antibody positive, the two most common subtypes of generalized MG. Rozanolixizumab-noli is a humanized IgG4 monoclonal antibody that is administered through a weekly subcutaneous infusion. A 6-week treatment cycle study has shown rapid improvement in clients’ activities of daily living (DailyMed, Rystiggo, 2023). Significant adverse effects include the increased risk of infection, aseptic meningitis, and hypersensitivity reactions, such as rash and angioedema. It is contraindicated in those with an active infection and should not be started until the infection is resolved (Source: DailyMed, Rystiggo, 2023).
Abnormally elevated blood level of thyroid hormones T3 and T4, often caused by a pituitary tumor, thyroid tumor, or autoimmune reaction in which antibodies overstimulate the follicle cells of the thyroid gland.
Introduction to Alzheimer's Disease
From Pharmacology for Nurses- Ch 10
Learning Objectives
By the end of this section, you should be able to:
- 10.3.1 Describe the pathophysiology of Alzheimer’s disease.
- 10.3.2 Identify the clinical manifestations related to Alzheimer’s disease.
- 10.3.3 Identify the etiology and diagnostic studies related to Alzheimer’s disease.
Alzheimer’s disease (AD) is the most common neurodegenerative condition of the brain and is characterized by significant changes in brain tissue. This disease is the most frequent cause of dementia in older adults. It is estimated that nearly 13 million Americans age 65 and older will develop AD and other dementias by 2050, according to the 2023 Alzheimer’s Association Disease Facts and Figures report. Alzheimer’s is irreversible and eventually has a major negative impact on cognition due to loss of short-term memory, reasoning, insight, and judgment and the inability to learn new information. It also has an undesirable effect on a client’s social functioning skills. However, the disease progresses very slowly; the risk of AD increases with age, but it can also occur between the ages of 30 and the early 60s (National Institute of Neurological Disorders and Stroke, 2023a).
Several factors have been identified that may reduce the risk of AD. These include higher levels of formal education, routinely engaging in mentally challenging activities—such as reading—adequate uninterrupted deep sleep, engaging in routine aerobic exercise, eating a healthy balanced diet, and maintaining social interactions.
Two major goals of care are maintaining socialization and providing support for caregivers. One way to provide relief for caregivers while increasing socialization for clients is adult daycare and respite centers.
Link to Learning
Alzheimer’s Prevention
https://www.youtube.com/watch?v=twG4mr6Jov0
In this TEDx Talk, entitled “What You Can Do to Prevent Alzheimer’s,” neuroscientist Lisa Genova describes the pathophysiology of the disease, with an emphasis on ways to potentially prevent the disease. Her presentation also discusses the concept of neuroplasticity and building a resistance to the changes in the brain seen with AD.
https://www.youtube.com/watch?v=xBDGgovA1LI
This video, also featuring Lisa Genova, explains that most forgetfulness in aging is normal. It also describes five ways to build an Alzheimer’s-resistant brain.
Pathophysiology
To fully understand how a drug alters symptoms, it is important to understand the pathophysiology of the disease being treated at the biochemical level. In most central nervous system disorders, the existing knowledge is limited. The brain is a complex structure, and the overall neuronal degeneration and cerebral atrophy recognized in AD has been theorized to result from a variety of changes. Researchers are still trying to unravel the underlying pathophysiology of AD. The following is a list of alterations that have been identified as origins for the cognitive decline seen with this disease (Huang, 2023):
- Degeneration of neurons: This destruction of neurons first occurs in the hippocampus, the area of the brain that plays an essential role in memory. The degeneration of these neurons will cause short-term memory loss. When neurons of the cerebral cortex begin to degenerate, speech, reasoning, and other higher cognitive functions become impaired.
- Beta-amyloid plaques: These plaques form outside neurons. Their central core is composed of beta-amyloid, a protein fragment of amyloid precursor protein (APP). Accumulation of beta-amyloid begins very early in the disease before any appearance of clinical manifestations. It is believed this protein plays a central role in AD.
- Neurofibrillary tangles and abnormal tau protein: These tangles form inside neurons. They result when the orderly arrangement of microtubules becomes disrupted. Microtubules are responsible for bringing nutrients to the axons and back. Normally, tau protein binds to these microtubules and provides stability. In AD, tau protein becomes “sticky” and tangles together with other tau threads. The microtubule is unable to transport nutrients, so the neuron can no longer function and eventually dies. As more and more neurons die, the brain atrophies.
- Oxidative stress: Oxidative stress produces reactive oxygen species (ROS), such as free radicals. These cause brain cell damage and cellular apoptosis. Oxidative stress is the term used to describe damage to cellular components caused by ROS. Due to their characteristic unpaired electrons, ROS can set off chain reactions where they remove electrons from other molecules, which then become oxidized and reactive and do the same to other molecules, causing a chain reaction. ROS can cause permanent damage to cellular lipids, proteins, carbohydrates, and nucleic acids. Damaged DNA can lead to genetic mutations and even cancer.
- Deficiency of ACh: The loss of ACh is crucial for two reasons: (1) it is an important transmitter in the hippocampus and cerebral cortex, where the degeneration is occurring; and (2) this transmitter is critical in forming memories.
- Genetics: Apolipoprotein E is known for its role in transporting cholesterol. One form of apolipoprotein E is associated with AD. Genetic research has shown that those with one or two copies of the gene that codes for apolipoprotein E4 (APOE-e4) are at a higher risk for developing AD. Additional genes have been identified as being definitively associated with AD. These include amyloid precursor protein (APP) gene, presenilin-1 (PS1) gene, and presenilin-2 (PS2) gene. A person who has any mutation to these genes will produce proteins that have neurotoxic properties, which will promote neuronal death. Additionally, these mutations can lead to the formation of neurofibrillary tangles and plaques.
Etiology
An underlying single cause for AD has yet to be discovered. There have been important theories and findings, but it is not known how these pieces fit together. Interestingly, the major pathologic findings begin to develop a decade or more before clinical manifestations are even observed. At this point, the etiology is considered multifactorial. Current potential causes of AD include:
- Degeneration of neurons in the hippocampus and cerebral cortex that subsequently cause cerebral atrophy
- Formation of beta-amyloid plaques
- Accumulation of neurofibrillary tangles and chemically altered tau protein
- Oxidative stress forming free radicals that damage cellular components caused by ROS (This is discussed more in the following section.)
- Deficiency of acetylcholine
- Genetics
Figure 10.3 compares a cross-section of a normal brain with one from a client with Alzheimer’s disease.
Although it is impossible to definitively diagnose AD without a postmortem autopsy, the diagnosis is made based on symptoms and exclusion of alternative pathology. During the autopsy, the typical characteristics of senile plaques and neurofibrillary tangles can be visualized.
Clinical Manifestations
As the disease progresses, the clinical manifestations worsen to the point where the client is unable to independently perform their activities of daily living (ADL). They become reliant on others for assistance. Early in the disease, the following mild manifestations may be witnessed: confusion, memory loss, disorientation, getting lost in familiar surroundings, problems with routine tasks, and changes in personality and judgment. Moderate manifestations include difficulty with ADLs (feeding and bathing), impaired organization and planning, impaired mathematical ability, anxiety/agitation, sleep disturbances, wandering, and difficulty in recognizing family and friends. Late manifestations include loss of speech, anorexia, impaired swallowing, weight loss, difficulty with movement, loss of ability to appropriately respond to the environment, sense of paranoia, delusions, hallucinations, and inability to control bladder and bowel function. At this point, the client is completely dependent on caregivers. AD will eventually destroy enough brain function to cause death.
Pharmacological Management
Currently, there is no known cure for AD. Drugs given for AD may at best slow the loss of memory and cognition in hopes of affording the person extra time to be able to continue functioning independently. Unfortunately, often the person observes minimal and short-term clinical efficacy from these medications. The AChE inhibitors (also known as cholinesterase inhibitors) were the first class of drugs approved by the FDA to treat AD. There are currently three drugs within this class. The other drug class, which currently contains one drug, is the N-methyl-D-aspartate (NMDA) receptor antagonist. In 2014, a capsule containing a combination of memantine hydrochloride ER (NMDA receptor antagonist) and donepezil hydrochloride (AChE inhibitor) was approved for the treatment of moderate to severe dementia of the Alzheimer’s type. This drug has the capability of targeting two different sites of action.
Next- 12.2.4 Alzheimer's Drugs
Access for free at https://openstax.org/books/pharmacology/pages/1-introduction
by OpenStax is licensed under Creative Commons Attribution License v4.
A disorder caused by an overproduction of PTH that results in excessive calcium resorption from bone, causing significantly decreased bone density and spontaneous fractures, decreased responsiveness of the nervous system, and calcium deposits in the body’s tissues and organs, impairing their functioning.

