1.1.1 Pharmacokinetics
Ernstmeyer & Christman - Open Resources for Nursing (Open RN)
Pharmacokinetics – Examining the Interaction of Body and Drug
Overview
Pharmacokinetics is the term that describes the four stages of absorption, distribution, metabolism, and excretion of drugs. Drugs are medications or other substances that have a physiological effect when introduced to the body. There are four basic stages a medication goes through within the human body: absorption, distribution, metabolism, and excretion. This entire process is sometimes abbreviated ADME.
Absorption is the first stage of pharmacokinetics and occurs after medications enter the body and travel from the site of administration into the body’s circulation. Distribution is the second stage of pharmacokinetics. It is the process by which medication is spread throughout the body. Metabolism is the third stage of pharmacokinetics and involves the breakdown of a drug molecule. Excretion is the final stage of pharmacokinetics and refers to the process in which the body eliminates waste. Each of these stages is described separately in the following sections of this chapter.
Research scientists who specialize in pharmacokinetics must also pay attention to another dimension of drug action within the body: time. Scientists do not have the ability to visualize where a drug is going or how long it is active. To compensate, they use mathematical models and precise measurements of blood and urine to determine where a drug goes and how much of the drug (or breakdown product) remains after the body processes it. Other indicators, such as blood levels of liver enzymes, can help predict how much of a drug is going to be absorbed.
Principles of chemistry are also applied while studying pharmacokinetics because the interactions between drugs and body molecules represent a series of chemical reactions. Understanding the chemical encounters between drugs and biological environments, such as the bloodstream and the oily surfaces of cells, is necessary to predict how much of a drug will be metabolized by the body.
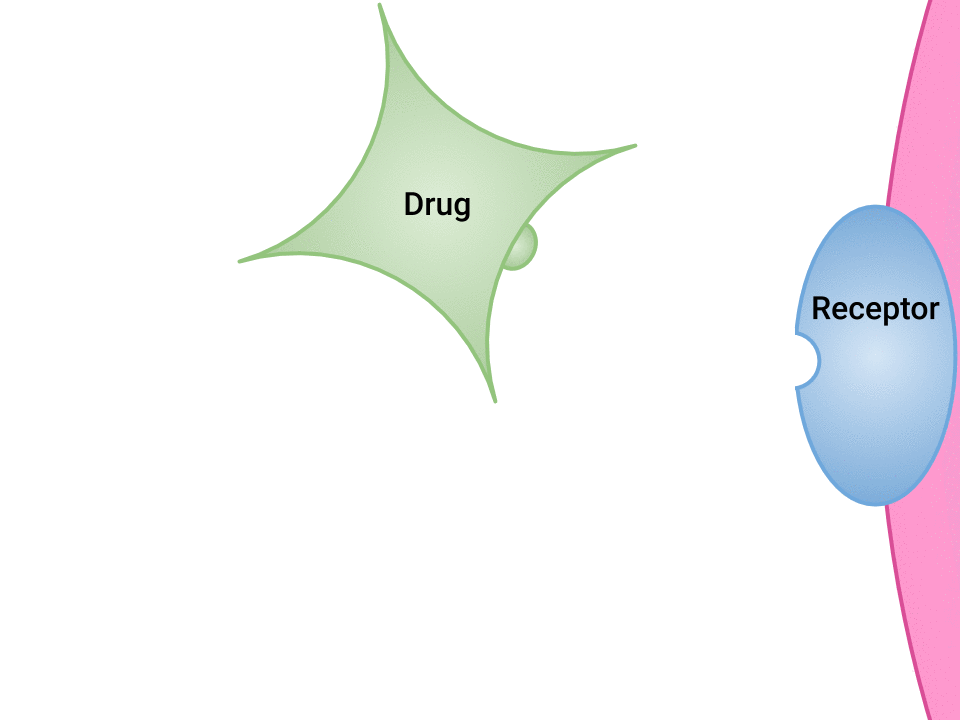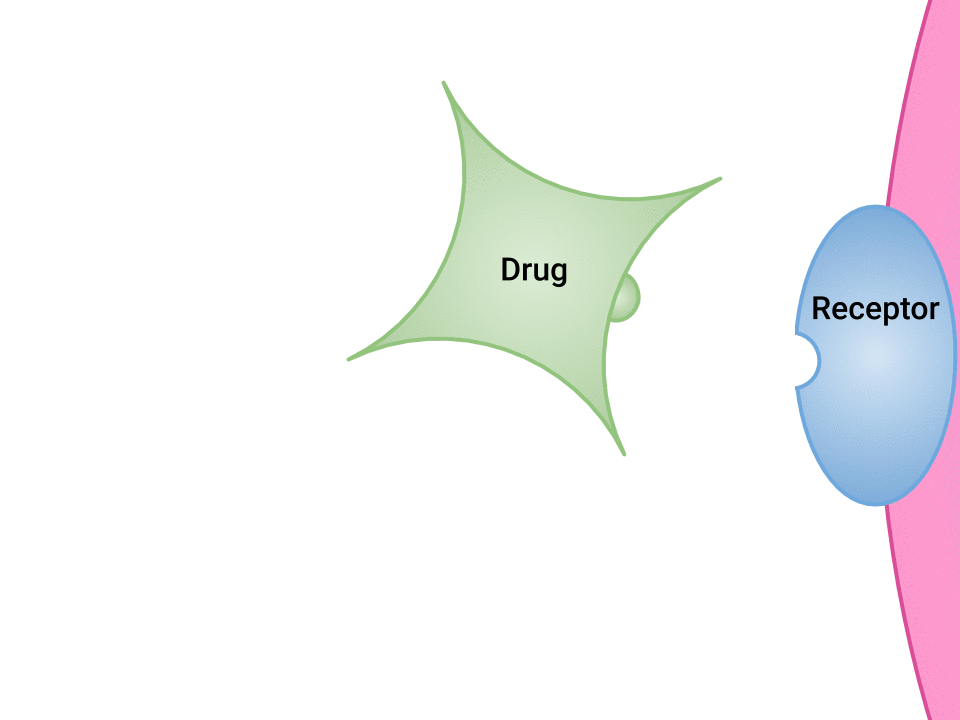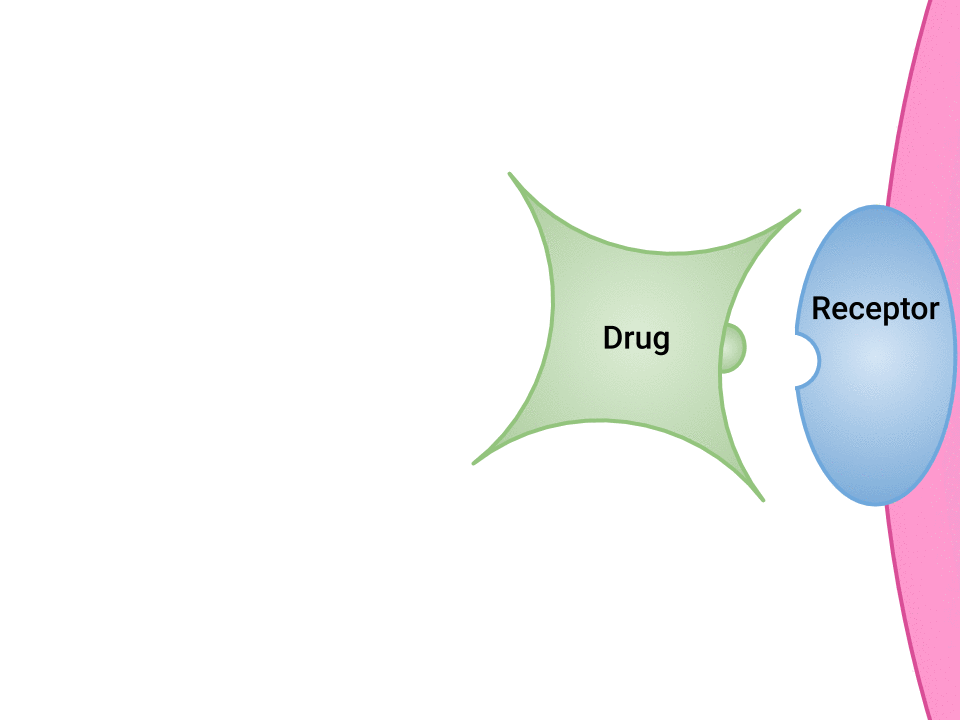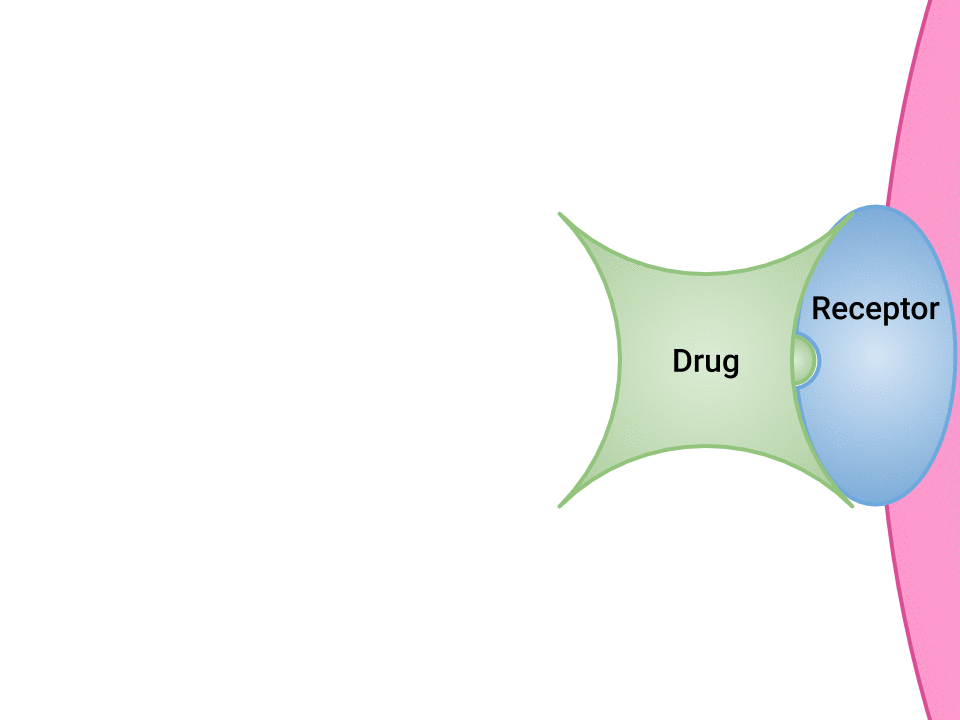
Pharmacodynamics refers to the effects of drugs in the body and the mechanism of their action. As a drug travels through the bloodstream, it exhibits a unique affinity for a drug-receptor site, meaning how strongly it binds to the site. Drugs and receptor sites create a lock and key system (see Figure 1.1[1]) that affect how drugs work and the presence of a drug in the bloodstream after it is administered. This concept is broadly termed as drug bioavailability.
The bioavailability of drugs is an important feature that chemists and pharmaceutical scientists keep in mind when designing and packaging medicines. However, no matter how effectively a drug works in a laboratory simulation, the performance in the human body will not always produce the same results, and individualized responses to drugs have to be considered. Although many responses to medications may be anticipated, a person’s unique genetic makeup may significantly impact their response to a drug. Pharmacogenetics is defined as the study of how people’s genes affect their response to medicines.[2]
Next- 1.1.2- Absorption
Media Attributions
- Drug Binding Barrier Animation
- “Drug and Receptor Binding” by Dominic Slausen at Chippewa Valley Technical College is licensed under CC BY 4.0 ↵
- This work is a derivative of Medicines by Design by US Department of Health and Human Services, National Institutes of Health, National Institute of General Medical Sciences and is available in the Public Domain. ↵
A condition where the body’s cells become resistant to the effects of insulin. Over time, the beta cells become exhausted and if blood glucose levels cannot be controlled through a healthy diet and exercise, then oral diabetic medication must be implemented and eventually insulin administration may be required.
Glucocorticoids and Mineralocorticoids
From Pharmacology for Nurses- Ch 6
Learning Objectives
By the end of this section, you should be able to:
- 26.4.1 Identify the characteristics of glucocorticoid and mineralocorticoid drugs used to treat adrenal disorders.
- 26.4.2 Explain the indications, actions, adverse reactions, contraindications, and interactions of glucocorticoid and mineralocorticoid drugs used to treat adrenal disorders.
- 26.4.3 Describe nursing implications of glucocorticoid and mineralocorticoid drugs used to treat adrenal disorders.
- 26.4.4 Explain the client education related to glucocorticoid and mineralocorticoid drugs used to treat adrenal disorders.
Glucocorticoids
Glucocorticoids and mineralocorticoids are two types of steroid hormones that are produced by the adrenal cortex and play distinct roles in regulating the body’s metabolism, fluid balance, and stress response. Glucocorticoids are produced in response to stress and help regulate metabolism and the immune system.
The primary glucocorticoid in the body is cortisol. Cortisol helps regulate glucose uptake in other tissues—such as muscles and fat—and has an anti-inflammatory and immunosuppressive effect, making it an important medication in the treatment of many inflammatory and autoimmune disorders and in preventing transplantation rejection. Nurses should note that there are many corticosteroids within this class of drugs, and they vary by potency, route of administration, and other non-anti-inflammatory activity. These properties help the health care provider choose the appropriate corticosteroid agent to prescribe for the client. Synthetic glucocorticoids—such as prednisone, dexamethasone, and hydrocortisone—are commonly used in medicine to treat conditions such as asthma, rheumatoid arthritis, and inflammatory bowel disease.
Although glucocorticoids can be effective at treating certain conditions, they can have significant toxic effects over time that limit use. As such, their use is typically reserved for short-term treatment of acute conditions or as a last resort for long-term conditions that have not responded to other treatments. Short-term use is tapered quickly to avoid rebound symptoms. Long-term use also requires tapering due to HPA axis suppression resulting in inadequate cortisol production.
Cortisone Acetate
Cortisone acetate is a glucocorticoid that also has salt-retaining properties. It is indicated for use with primary or secondary adrenocortical insufficiency, hypercalcemia associated with cancer, rheumatic disorders, collagen disorders, allergic states, inflammatory bowel disorders, and various respiratory, hematologic, integumentary, and neoplastic disorders. Adverse effects include fluid retention, sodium retention, hypertension, muscle weakness, osteoporosis, headache, and weight gain.
Hydrocortisone
Hydrocortisone, a synthetic form of cortisol, is a short-acting glucocorticoid that is used to reduce inflammation and swelling in the body. Hydrocortisone is used to treat a variety of conditions including skin disorders, allergic reactions, asthma, arthritis, and certain types of cancer. It can be applied topically, as a cream or ointment, and has ophthalmic, otic, and rectal routes available. Adverse effects include headache, dizziness, nausea, weight gain, mood changes, increased blood pressure, hyperglycemia, difficulty sleeping, dry skin, acne, skin irritation, and increased risk of infections.
Clinical Tip
Hydrocortisone Administration
Systemic administration of steroids is often recommended to take place in the morning to avoid insomnia (Hodgens & Sharman, 2023).
Methylprednisolone
Methylprednisolone is an intermediate-acting synthetic glucocorticoid that is used as an anti-inflammatory and immunosuppressant. It is commonly used to treat a variety of inflammatory and autoimmune conditions. Methylprednisolone—available in oral, injectable, and topical forms—works by binding to glucocorticoid receptors in the cells of the body, which leads to a reduction in the release of inflammatory molecules, such as cytokines, and inhibits the immune response and decreases inflammation. Dosing is based on the condition being treated and the severity of symptoms.
Prednisolone
Prednisolone, an intermediate-acting synthetic form of cortisol, is used to decrease inflammation and to treat a variety of conditions including rheumatic conditions, allergic reactions, asthma, and certain types of cancer. The medication comes in an oral form and as an injectable. Adverse effects include increased glucose levels, injection site reactions, weight gain, and mood changes.
Prednisone
Prednisone is an intermediate-acting steroid medication and a synthetic form of cortisol used to reduce inflammation. Like prednisolone, it is used in the treatment of a variety of conditions including asthma, allergic reactions, rheumatic disorders, respiratory disorders, and certain types of cancer. Adverse effects include weight gain, mood changes, increased blood sugar levels, and osteoporosis.
Betamethasone
Betamethasone is a long-acting synthetic cortisol that reduces inflammation and swelling in the body and is used to treat various disorders including skin and inflammatory disorders such as rheumatoid arthritis. Adverse effects include skin irritation, increased risk of infection, and elevated blood glucose levels.
Dexamethasone
Dexamethasone is a long-acting synthetic form of cortisol that is used to reduce inflammation in the body and may also be used as an immunosuppressant. Dexamethasone, which comes in an oral and injectable form, is used to treat numerous disorders including rheumatic, respiratory, asthma, and allergic reactions. This drug is also used in the diagnosis of Cushing’s syndrome as a suppressive test. Adverse effects include increased risk of infection, weight gain, irritability and mood changes, increased blood sugar levels, GI upset, and osteoporosis.
Table 26.6 lists common glucocorticoids and typical routes and dosing for adult clients.
| Drug | Routes and Dosage Ranges |
|---|---|
| Cortisone acetate (Cortison, Cortisyl) |
Typical dose: 25–300 mg orally daily depending on the specific disease entity being treated. |
| Hydrocortisone (Cortisporin, Cortizone, Solu-Cortef, Proctocort) |
Topical cream: Depends on formulation, typically 1%, 3–4 times daily. IV: 100–500 mg; dose extremely variable based on client and indication. |
| Methylprednisolone (Medrol, Solu-Medrol) |
Typical dose: 4–200 mg daily depending on the specific disease entity being treated. Routes include oral (dose-pack, tablets, solution), intravenous, intra-articular, and intramuscular. |
| Prednisolone (Millipred, Omnipred, Prelone) |
Typical dose: 5–60 mg orally daily depending on the specific disease entity being treated. |
| Prednisone (Deltasone) |
Typical dose: 5–60 mg orally daily depending on the specific disease entity being treated. |
| Betamethasone (Celestone, Betaject) |
Typical dose for injectable: 0.25–0.9 mg/day intravenously or intramuscularly. |
| Dexamethasone (Decadron, Dexasone) |
Typical dose: 0.5–9 mg daily depending on the specific disease entity being treated. Routes include oral, intravenous, intra-articular, and intramuscular. |
Adverse Effects and Contraindications
Short-term adverse effects include insomnia, hunger, and mood changes. Long-term adverse effects include weight gain, high blood pressure, osteoporosis, hyperglycemia, skin atrophy (topical application), iatrogenic Cushing’s syndrome, and increased risk of infection.
Glucocorticoids are contraindicated in clients with hypersensitivity to any component of the formulation, those who have an active systemic fungal infection, or those with uncontrolled hyperglycemia.
Table 26.7 is a drug prototype table for glucocorticoids featuring methylprednisolone. It lists drug class, mechanism of action, adult dosage, indications, therapeutic effects, drug and food interactions, adverse effects, and contraindications.
| Drug Class Synthetic glucocorticoidMechanism of Action Binds to glucocorticoid receptors, decreasing the release of inflammatory molecules and inhibiting the immune response |
Drug Dosage Typical dose: 4–200 mg daily depending on the specific disease entity being treated. Routes include oral (dose-pack, tablets, solution), intravenous, intra-articular, and intramuscular. |
| Indications Inflammatory conditions Autoimmune disordersTherapeutic Effects Decreases inflammation Suppresses the immune response |
Drug Interactions Oral anticoagulants AspirinFood Interactions No significant interactions |
| Adverse Effects Weight gain High blood pressure Osteoporosis Increased risk of infection Skin atrophy Hyperglycemia/glycosuria |
Contraindications Hypersensitivity Systemic active fungal infectionsCaution: May increase risk of infection |
Mineralocorticoids
Mineralocorticoids are a type of steroid hormones that are produced by the adrenal cortex and are involved in regulating the body’s fluid balance and electrolyte levels. The primary mineralocorticoid in the body is aldosterone.
Disorders of mineralocorticoid production or activity can lead to imbalances in fluid and electrolyte levels: two such conditions exist. The first is hyperaldosteronism, which is characterized by excess aldosterone production. An excess of aldosterone causes an increase in blood pressure and serum sodium levels along with a decrease in serum potassium levels. The other condition is hypoaldosteronism, which is characterized by insufficient aldosterone production. Hypoaldosteronism leads to a decrease in blood pressure and serum sodium levels along with an increase in serum potassium levels.
Aldosterone
Aldosterone works by binding to mineralocorticoid receptors in the cells of the kidneys, which increases the reabsorption of sodium ions and the excretion of potassium ions. This leads to an increase in blood volume and blood pressure. In addition to regulating fluid and electrolyte balance, aldosterone plays a role in the renin-angiotensin-aldosterone system (RAAS). The RAAS is a complex hormonal system that helps to regulate blood pressure by controlling the balance of sodium and water in the body.
Fludrocortisone
Fludrocortisone is a synthetic adrenocortical steroid that binds to mineralocorticoid receptors in the cells of the kidneys and supports fluid and electrolyte balance within the body. This medication is used to treat primary and secondary adrenocortical insufficiency in Addison’s disease. Adverse effects include hyperglycemia, hypokalemia, hypertension, muscle weakness, and impaired wound healing. It is contraindicated in clients with fungal infections and hypersensitivity.
Adverse Effects and Contraindications
Common adverse effects of mineralocorticoid drugs include fluid retention because they promote sodium and water reabsorption in the kidneys; hypokalemia where symptoms include muscle weakness, fatigue, irregular heartbeat, and muscle cramps; elevated blood pressure; gastrointestinal disturbances such as stomach discomfort, nausea, and diarrhea; and mood and behavioral changes such as mood swings, irritability, or anxiety.
Contraindications include hypersensitivity to the drug or any of its components, systemic fungal infections, heart failure, and severe kidney impairment. Mineralocorticoids can suppress the immune system and worsen systemic fungal infections; heart failure is contraindicated because these drugs can cause fluid retention and lead to worsening of fluid volume overload; and severe kidney impairment can occur in clients with kidney disease as these drugs are excreted through the urine and can cause further renal insufficiency.
Table 26.8 is a drug prototype table for mineralocorticoids featuring fludrocortisone acetate. It lists drug class, mechanism of action, adult dosage, indications, therapeutic effects, drug and food interactions, adverse effects, and contraindications.
| Drug Class Mineralocorticoid
Mechanism of Action |
Drug Dosage For salt-losing adrenogenital syndrome: Typical dose: 0.1–0.2 mg orally daily. For Addison’s disease: Usual dose: 0.1 mg orally daily, although dosage ranging from 0.1 mg 3 times a week to 0.2 mg daily has been employed. |
| Indications Primary and secondary adrenocortical insufficiency in Addison’s disease Treatment of salt-losing adrenogenital syndrome
Therapeutic Effects |
Drug Interactions Amphotericin B Potassium-depleting diuretics Digitalis glycosides Oral anticoagulants Antidiabetic drugs Aspirin Barbiturates Phenytoin Rifampin Anabolic steroids Vaccines Estrogen
Food Interactions |
| Adverse Effects Edema Cardiac enlargement Potassium loss Muscle weakness Abdominal distention Impaired wound healing Growth suppression Hyperglycemia Urticaria |
Contraindications Systemic fungal infections Hypersensitivity
Caution: |
Nursing Implications
The nurse should do the following for clients who are taking glucocorticoids and mineralocorticoids:
- Educate the client regarding glucocorticoid and mineralocorticoid therapy.
- Assess the client’s knowledge about signs and symptoms of over- and undertreatment, adverse reactions, and contraindications and clarify any gaps in knowledge.
- Monitor vital signs and electrolytes, especially sodium and potassium, renal function, glucose levels, serum aldosterone levels, serum cortisol levels, and bone density test, closely.
- Report any abnormalities or symptoms such as weight gain, edema, crackles, jugular vein distention, fever, tachycardia, tachypnea, shortness of breath, or a change in mental status to the health care provider.
- Provide client teaching regarding the drug and when to call the health care provider. See below for additional client teaching guidelines.
Client Teaching Guidelines
The client taking a glucocorticoid or mineralocorticoid should:
- Take drugs as prescribed by their health care provider.
- If using the drug systemically, avoid people who are sick—especially those who have chickenpox, measles, or tuberculosis—because they are at an increased risk of contracting the condition.
- Report signs of fluid volume overload such as a weight gain of 2 pounds in 3 days or 5 pounds in a week.
- Limit salt intake.
- Report symptoms of infection, such as a fever greater than 100.4°F, a fast heartbeat, fast breathing, and symptoms of electrolyte imbalances such as weight gain, edema, a change in mental status, or shortness of breath and symptoms of cardiovascular issues including jugular vein distention, tachycardia, and/or palpitations to the health care provider.
The client taking a glucocorticoid or mineralocorticoid should not:
- Stop taking this medication without consulting with the health care provider because this drug must be tapered to decrease adverse effects.
FDA Black Box Warning
Corticosteroids
Injection into the epidural space of the spine may result in rare but serious adverse events including loss of vision, stroke, paralysis, and death.
Next- 8.4 Introduction to HIV, AIDS, and Antiretrovirals
Access for free at https://openstax.org/books/pharmacology/pages/1-introduction
by OpenStax is licensed under Creative Commons Attribution License v4.
Introduction to Cancer and Phases of Cancer Therapy
From Pharmacology for Nurses- Ch 8
Learning Objectives
By the end of this section, you should be able to:
- 8.1.1 Describe cancer and cancer development.
- 8.1.2 Discuss contributing theories of environmental versus genetic etiologies of cancer development.
- 8.1.3 Identify the characteristics of cancer cells.
- 8.1.4 Describe different types of cancer.
Cancer Development
Each day, the human body experiences cell mutations that result in a change in cellular structure, which may lead to cancer development. When this happens, the intracellular machinery may be able to repair the mutation, or the body’s immune system recognizes these cells as abnormal and attacks them. These are two innate defenses against cancer development. When either defense fails, cancer development can occur. There are multiple theories about the causes of cancer development. The most common theories are based on environmental exposures and genetic predispositions as causes of cancer development.
Environmental and Genetic Factors
Environmental exposures are outside factors that can cause cell mutations when the body is exposed to them. Most commonly, these factors include exposure to tobacco products, benzenes, petroleum-based products, asbestos, and certain drugs, including chemotherapies. There are many other substances that are classified as carcinogens, or cancer-causing agents. Somatic mutations occur from carcinogen exposures after birth. They do not develop from genetic mutations and do not affect germ cells that become ova and sperm.
Link to Learning
Environmental Exposure in a Community: Love Canal, New York
https://youtu.be/TUTF57Chos4?si=1B6WPckISSCxzz80
One of the earliest findings of cancer and other diseases directly linked to environmental exposures occurred in the small community of Love Canal, Niagara Falls, in upstate New York. The story of Love Canal, while very unfortunate, brought about widespread change in environmental protection and regulation of toxic substances.
Genetic factors are those internal predispositions to cellular changes that result in cancer development. For example, clients who inherit the breast cancer oncogenes (BRCA1 and BRCA2) have a much higher chance of developing breast cancer than clients without these mutations (Centers for Disease Control and Prevention [CDC], 2020). These are considered to be germline mutations that develop in the eggs or sperm of a parent and are passed on to offspring.
Link to Learning
Oncogenes and Breast Cancer
Because genetic mutations that cause specific cancers to develop are present at birth, screening for the presence of some of these oncogenes is now possible. When these oncogenes are identified before cancer has developed, clients now have options for improved surveillance and early detection and intervention. This promotes better outcomes for those clients who were born with hereditary gene mutations. The breast cancer antigen mutations 1 and 2 (BRCA1, BRCA2) are two of the most well-understood oncogenes.
Additional theories are based on immune system failure and the effects that physical and mental stress have on the body’s ability to defend itself. Cell mutations occur frequently throughout the human body, but these cells are usually targeted and either repaired or destroyed by the immune system. When the immune system fails to recognize mutated cells, these cells continue replication, forming a tumor. Other illnesses, physical stress, and mental distress are some factors that decrease the effectiveness of the immune-system functions. When considering theories of cancer development, most researchers agree that a combination of causative factors, rather than one single theory, contributes to cancer development (Mbemi et al., 2020).
Cellular Changes
Once a cancer-causing mutation occurs, if it is unable to be repaired, the mutated cells continue to divide, resulting in the development of a tumor or malignancy (see Figure 8.2). These cells lose many of the regulatory characteristics of normal cells, including contact inhibition and a regulated rate of mitosis. Tumors also have the property of neoangiogenesis, which is the ability to grow new blood vessels to support the metabolic needs associated with the abnormal growth. As these cells continue to grow, they can result in physical changes that greatly affect the function of the body.
Pain, compression, nutritional deficiencies, weight loss, and fluid and electrolyte imbalances are some of the effects that can occur with cancer development. However, tumors can also go unnoticed for many years. As growth continues, cancerous cells can metastasize to distant sites through direct extension into surrounding tissues, through seeding, and by embolization into the lymph and circulatory systems.
Cancer Types
Cancers are classified either by the type of tissue in which the cancer originates (histological type) or by the location in the body where the cancer originated (primary site) (National Cancer Institute, n.d.). Health care providers tend to refer to a cancer’s histological type, while clients more frequently refer to the cancer’s location. Many types of cancer will form tumors. Solid tumors are cancers that form in tissues of bones, skin, organs, and muscles. Liquid, or hematologic, tumors arise in the bone marrow and involve blood and lymphatic cells. Typically, regardless of tumor type, pharmacologic treatment will involve medications with systemic effects.
Solid Tumors
Solid tumors develop as a mass of cancer cells that originate in a specific area of the body. Adenocarcinomas and squamous cell carcinomas are two common pathological types of solid tumors. Adenocarcinomas develop in glandular and epithelial tissues, while squamous cell carcinomas arise in the skin or in the lining of the respiratory and gastrointestinal system (National Cancer Institute, n.d.). Another type of solid tumor that is less common is a sarcoma. These tumors may form in soft tissues including fat, muscles, nerves, vessels, and skin. Sarcomas may also form in bones and most often affect young adults. In the United States, lung, prostate, and breast cancers are the leading causes of cancer-related mortality (National Cancer Institute, n.d.).
Hematologic (Liquid) Tumors
Hematologic cancers are cancers that arise in the bone marrow and involve blood cells, especially white blood cells and their precursors, and lymphocytes. These cancers are often called liquid tumors because they originate in the bone marrow and flow in the bloodstream. In the United States, non-Hodgkin lymphoma, leukemia, and multiple myeloma are the three most common hematologic cancers, respectively. These types of tumors present multiple issues because they exist in the vascular system, circulating through all parts of the body. When these cells are destroyed by chemotherapy, cellular debris and electrolytes are released into the blood. This may result in tumor lysis syndrome (TLS), a life-threatening condition that is characterized by acidosis, hyperkalemia, hyperphosphatemia, and hypocalcemia. When chemotherapy is given for a blood tumor in which high numbers of cancer cells are in the blood, clients must be pretreated with hydration therapy, allopurinol or rasburicase, and management of electrolytes. While tumor lysis occurs most commonly in hematologic cancers, it may occasionally occur with solid tumors that are very large and bulky.
Link to Learning
Tumor Lysis Syndrome
Tumor lysis syndrome is considered an oncologic or medical emergency, which without immediate recognition and treatment can result in fatal outcomes. The Cleveland Clinic has provided important updates about this syndrome that can occur within hours of cancer treatment.
Next- 8.5.2 Chemotherapeutic Drugs
Access for free at https://openstax.org/books/pharmacology/pages/1-introduction
by OpenStax is licensed under Creative Commons Attribution License v4.
Chemotherapeutic Drugs
From Pharmacology for Nurses- Ch 8
Learning Objectives
By the end of this section, you should be able to:
- 8.2.1 Identify principles of proper handling of chemotherapeutic agents.
- 8.2.2 Discuss different administration methods, phases, routes of administration, and types of chemotherapy.
- 8.2.3 Describe major side effects of chemotherapeutic agents.
- 8.2.4 Identify characteristics of different classes of chemotherapeutic agents used in cancer treatment.
- 8.2.5 Explain the indications, actions, adverse reactions, and interactions of chemotherapeutic agents used in cancer treatment.
- 8.2.6 Describe the nursing implications of chemotherapeutic agents.
- 8.2.7 Explain the client education related to chemotherapeutic agents.
Handling of Chemotherapeutic Agents
The complexity of administering chemotherapy and caring for clients that have been treated with cancer therapies requires advanced knowledge. Special training and certifications to administer chemotherapy are required. Chemotherapy agents may cause cytotoxic exposure to those who compound and administer these drugs as well as to clients, families, and other caretakers who might be exposed through spills, improper handling and disposal, and other means. Proper training in compounding, administering, and managing exposure emergencies is a requirement of all personnel who work with chemotherapy. Principles of appropriate handling include, but are not limited to, storage of cytotoxic substances in impervious containers, double gloving (chemotherapy-rated gloves), compounding in a negative-pressure hood, and disposing of cytotoxic substances based on national standards and institutional policies.
Trending Today
Chemotherapy Administration Safety Standards
The Oncology Nursing Society, a leader in developing oncology standards, developed chemotherapy administration guidelines and safety standards to assist nurses in safely administering chemotherapy and other cancer treatments. Advances in technology, cancer treatment, and nursing training prompted the need for a periodic review and revision of the standards for general oncology practices. The latest standards include pediatric oncology practices and new standards affecting chemotherapy prescription, preparation, and administration (Oncology Nursing Society, 2023).
Cancer Treatments
Chemotherapy may be given for many different reasons. Often, it is given as a curative measure to eradicate all malignant cells. However, cure is not always realistic. When cure is not attainable, chemotherapy can be used to decrease tumor size and prevent metastasis, and sometimes it may be used for palliation and symptom control. Differing regimens of chemotherapy are used for varying client needs (see Table 8.1).
| Type of Therapy | Description |
|---|---|
| Adjuvant therapy | Given after initial treatment with surgery to destroy leftover cells |
| Neoadjuvant therapy | Initial treatment given to shrink the cancer before surgery |
| Salvage therapy | Second-line therapy given when first-line therapy is unsuccessful |
| Targeted therapy | Given to selectively kill cancer cells without harming normal cells |
| Biologic therapy | Used to enable the immune system to better kill cancer cells |
Link to Learning
Chemotherapy Treatment
Phases of Chemotherapy
There are three phases of chemotherapy, beginning with induction. This phase is also known as first-line, front-line, or primary therapy. During this phase, the goal is to induce a remission. For most tumors, this may be the only phase required. For hematological cancers, clients may undergo a second phase, called consolidation, intensification, or post-remission therapy. This phase is used after a remission has been achieved, with a goal of eradicating any remaining cancer cells. The third phase, the maintenance phase, may be used either after induction or after consolidation. In this phase, a maintenance dose of chemotherapy is given to prevent reoccurrence of cancer. This phase is the longest phase and may last for several years.
Routes of Chemotherapy Administration
Chemotherapy may be administered by different routes depending on the purpose and toxicities of each individual drug. These different routes include oral, intravenous, subcutaneous, intramuscular, intracavitary, topically, and intrathecally. Intracavitary administration involves the infusion of a chemotherapeutic agent into a body cavity such as the bladder or abdomen. Intrathecal administration involves instilling chemotherapy into the spinal column or intracranially to treat cancers in the central nervous system. For drugs that are vesicants, which have the ability to cause necrosis if they extravasate, the intravenous route must be used. When a vesicant drug is being administered, slow administration in a rate/minute modality and vigilant assessment of the intravenous infusion site must be performed. Maintaining patency of the administration site is paramount to preventing tissue injury (see Figure 8.3).
Clinical Tip
Administering Vesicant Drugs
When administering a vesicant drug, the nurse should use extreme caution. If, for any reason, the nurse feels that an intravenous site could be compromised, the infusion should be immediately stopped and a new access obtained. Should an actual extravasation occur, the health care provider should be notified and any applicable protocols should be followed.
Chemotherapy may be given by different routes, depending on the cancer location. When cancer develops in a body cavity or in the brain or spinal column, treatment may require the use of special routes of administration to overcome the blood-brain barrier or to increase effectiveness by placing drug therapy more directly at the tumor site. Table 8.2 describes the different methods of chemotherapy administration.
| Method of Chemotherapy Administration | Description |
|---|---|
| Oral | Tablets, capsules, or liquids that are swallowed |
| Intravenous | Liquid preparations administered into a vein via peripheral or central catheters |
| Injection | Liquid preparations administered via intramuscular or subcutaneous injections |
| Intrathecal | Injected by syringe or catheter directly into the brain or spinal cord |
| Intraperitoneal | Injected or infused via catheter into the peritoneal cavity |
| Intracavitary | Injected or infused via catheter into a body cavity such as the bladder |
| Intraarterial | Injected directly into an artery flowing to tumor sites |
| Topical | Applied directly to the skin or tumor site |
Types of Chemotherapy
There are many different classifications of chemotherapy drugs, many of which fall into one of two broad categories—cell-cycle specific and cell-cycle nonspecific therapies. Each cell goes through five phases of mitosis before producing daughter cells (see Figure 8.4). Chemotherapy agents that cause cancer cell death in all phases of mitosis are referred to as cell-cycle nonspecific (CCNS), whereas those that are effective only in one phase of mitosis are called cell-cycle specific agents (CCS). In combination chemotherapy, both CCS and NCCS agents are used to eliminate cancer cells more completely.
Side Effects of Chemotherapy
Side effects of chemotherapy vary from client to client and drug to drug. However, most chemotherapies have overlapping side effects. Each of these side effects must be managed appropriately to protect the client and promote positive outcomes. Nurses who care for clients receiving chemotherapy must possess excellent assessment skills to recognize and treat these side effects early.
Most chemotherapies work by interrupting DNA or RNA synthesis. These chemicals are most effective in eliminating cells that are most rapidly reproducing, with high mitotic rates. Unfortunately, chemotherapy cannot tell the difference in cells that are abnormally dividing versus normally rapidly dividing; therefore, the client may experience effects on normal cells as well. This includes hair, nails, skin, mucus membranes, the lining of the gastrointestinal system, and, most importantly, the stem cells within the bone marrow.
Major Side Effects
Most chemotherapy agents have major side effects in common. These include myelosuppression, alopecia, nausea and vomiting, skin or nail changes, and late-term effect, including secondary cancers. Myelosuppression is one of the most common effects of chemotherapy. This occurs as chemotherapy affects the stem cells in the bone marrow, resulting in decreased production of white blood cells, red blood cells, and platelets. Myelosuppression affecting red blood cells, white blood cells, and platelets all together is referred to as pancytopenia. Without proper support during this myelosuppression, clients may experience sepsis, bleeding, and hypoxemia, which may result in increased mortality rates. Frequent monitoring of the client’s complete blood count is imperative. In some regimens, pancytopenia may happen very rapidly, in just a few days, while with other regimens, myelosuppression may not occur for 7–14 days or longer. The nadir period, or lowest point of myelosuppression, differs for each specific chemotherapy. Clients must be monitored closely after receiving myelosuppressive therapies for the nadir period, which predisposes clients to safety issues including infection, bleeding, impaired oxygenation, and falls. Protective measures must be instituted to prevent these from occurring, and the nurse must teach clients self-care principles (see Client Teaching Guidelines).
Client Teaching Guidelines
To prevent infection, the client receiving chemotherapy should:
- Bathe daily to reduce bacterial colonization.
- Cleanse their mouth after each meal and at bedtime.
- Cook eggs, meats, and seafood thoroughly to decrease the risk of foodborne illness.
- Use gloves when gardening to protect hands from direct contact with soil, which can contain bacteria and mold.
- Increase dietary fluids and fiber.
- Notify their health care provider for a temperature greater than 100.4°F (38°C), productive cough, diarrhea, or urinary burning, urgency, or frequency.
To prevent infection, the client receiving chemotherapy should not:
- Eat raw fruits and vegetables to decrease the risk of developing a foodborne illness.
- Eat wild game such as deer, rabbits, and pheasants to decrease the risk of ingesting contaminated meat.
- Clean cat litter boxes to decrease the risk of contact with bacteria or parasites.
Neutropenia, leukopenia, and granulocytopenia occur when the white cell indices (neutrophils, leukocytes, granulocytes) are decreased. This is typically defined as having an absolute neutrophil count less than 1.5 cells/mcL. When this occurs, the client may be prescribed prophylactic antibiotic therapy that may include broad-spectrum gram-positive, broad-spectrum gram-negative, and/or antifungal agents to assist in preventing sepsis. (See Anti-infective Drugs.) Clients with neutropenia present a fragile and complicated situation, especially in regard to nursing care and assessments. It is common for severely neutropenic clients to acquire infections, and even sepsis, with few clinical symptoms. Vigilant nursing assessment is a priority in preventing negative client outcomes. With low white blood cell counts, normal immune responses to infection may be absent. For example, a client who has developed a local infection at a central venous access site would normally display warmth, redness, and edema at the site. However, that same client, when experiencing neutropenia, would not exhibit these symptoms. During this time of decreased immunity, clients may also be treated with biologic colony stimulating agents such as filgrastim or pegfilgrastim. These drugs stimulate increases in stem cell production of white blood cells, accelerating a client’s recovery from white blood cell destruction.
Clinical Tip
Febrile Neutropenia
Febrile neutropenia occurs when a client has an absolute neutrophil count that is less than 1.5 per microliter, accompanied by a body temperature above 100.4ºF. When it occurs, febrile neutropenia should become first priority for assessment and intervention. The health care provider should be called immediately. Typically, blood cultures will be obtained, and the client will begin empiric broad-spectrum antibiotics. Failure to recognize and treat febrile neutropenia may result in life-threatening sepsis.
Thrombocytopenia, another result of myelosuppression, results in increased risk of bleeding as the number of circulating platelets (thrombocytes) decreases. Thrombocytopenia may cause gastrointestinal hemorrhage, intracerebral bleeding, and other sites of bleeding such as gum and scleral hemorrhages. Platelet counts less than 50,000 per microliter require special precautions; below 20,000, the bleeding risk greatly increases, and the client may require platelet transfusions.
Client Teaching Guidelines
To prevent bleeding, the client being treated for thrombocytopenia should:
- Use tooth sponges for oral care to decrease the risk of bleeding secondary to brushing. Avoid alcohol-based mouthwash, which can dry the mouth and increase the risk of bleeding.
- Increase dietary fluids and fiber.
- Use stool softeners and fiber laxatives to produce regular, soft bowel movements.
To prevent bleeding, the client being treated for thrombocytopenia should not:
- Participate in contact sports or other activities that could cause bleeding.
Erythrocytopenia (anemia), a decrease in the production of red blood cells (erythrocytes), is another result of myelosuppression. Red blood cells function to carry oxygen to tissues, remove carbon dioxide from tissues, and provide volume within the intravascular space. When red blood cell counts decrease, clients may become hypoxemic when there are not enough red blood cells to transport oxygen. This results in fatigue and shortness of breath. Hypovolemia also occurs, in which decreased numbers of circulating red blood cells cause lowered vascular pressure. Clients may then experience orthostatic hypotension, dizziness, and disequilibrium. Clients may require transfusions of packed red blood cells, hydration with isotonic intravenous fluids, and oxygen. To decrease the occurrence of erythrocytopenia, clients may be given biologic colony stimulators to increase red blood cell production. These include epoetin alfa and darbepoetin.
Client Teaching Guidelines
To promote oxygenation, the client with anemia (erythrocytopenia) should:
- Schedule activities around periods of rest.
- Notify the health care provider if shortness of breath increases or does not resolve with rest.
- Sit on the side of the bed or chair for a few minutes before rising.
To promote oxygenation, the client with anemia (erythrocytopenia) should not:
- Perform tasks or engage in activities that cause shortness of breath.
Alopecia, or hair loss, is a side effect of some chemotherapies. Drugs such as doxorubicin damage hair follicles, which results in partial or complete hair loss. While this is not usually permanent, it will last throughout the duration of the treatments. Although this is not harmful for the client physically, it may be very difficult for a client emotionally and socially. Hair loss ultimately affects body image and self-esteem and may cause others to be uncomfortable around the client. Regrowth of hair usually begins within 6 months after treatments have finished (American Cancer Society, 2021).
Skin and nail changes may occur with administration of some types of cancer treatments. Skin rashes (erythema) or peeling of the skin on the hands and feet may occur. Hyperpigmentation of the skin and nail beds may develop as well. Tactile effects may cause neuropathic pain or increased sensitivity to cold. Nails may break, crack, or fall off. The nurse must provide care to prevent the development of infection should the nails or skin become broken.
Chemotherapy-associated nausea and vomiting is another common side effect and can vary from drug to drug and client to client. Risk factors for chemotherapy-induced nausea and vomiting include female sex, clients who experienced nausea and vomiting during pregnancy, age less than 50 years, and incidence of nausea and vomiting with previous chemotherapy treatments. Dehydration and electrolyte imbalances are also associated with increased nausea and vomiting. While there is pharmacological support for nausea and vomiting, some chemotherapeutic agents continue to cause significant negative effects. Nausea and vomiting may occur as anticipatory, beginning before a treatment begins, or during or soon after a treatment. Some agents like cisplatin may cause delayed nausea and vomiting, defined as occurring more than 24 hours after administration. This may last 5 days or more. Management of chemotherapy-induced nausea and vomiting includes efforts to maintain client comfort, adequate hydration, and electrolyte balance. Antiemetics such as ondansetron and aprepitant may be given before, during, and after treatment. Nutritional support to help maintain adequate intake of required nutrients and prevent chemotherapy-related cachexia (“wasting syndrome”) accompanies the management of nausea and vomiting.
For clients undergoing chemotherapy, another complication involves long-term effects. Most chemotherapy treatments effectively begin both therapeutic and untoward effects as soon as they are administered. The acute effects may last throughout the treatment period and for a few months afterward. However, the cumulative effects of repeated cycles of treatment often result in effects that persist and even worsen over many years. Long-term effects typically involve decreased pulmonary and cardiac function, but other effects such as infertility may be seen.
In addition to monitoring for late-term effects of chemotherapies, clients must be evaluated for recurrence of disease and for development of secondary cancers. Recurrence of a tumor happens when cells of the original cancer return and begin growing despite treatment modalities. Secondary cancers differ from recurrent cancer in that these tumors develop in different tissues than the original cancer. Chemotherapeutic agents, unfortunately, are carcinogens. Receiving chemotherapeutic agents for cancer treatment places a client at risk of developing other types of cancers known as secondary cancers. For cancer survivors, follow-up care includes continued surveillance to assess for these effects.
Alkylating Drugs
Alkylating agents are among the oldest group of chemotherapies. They were developed after World War I when it was noted that these substances, when used as weapons, caused myelosuppression. Upon this discovery, they were developed into chemotherapeutic agents. In general, the medications in this class kill cancer cells by cross-linking DNA, which which prevents cell division in a manner that can lead to toxicity within the cell or unbalanced growth, both of which can cause cell death. These agents work most effectively on slower-growing tumors with a slowed mitotic rate. Alkylating agents are separated into several different subclasses. Some of these medications are dosed based on the client’s weight, and others are administered based on the client’s body surface area (BSA), which is determined using a formula using the client’s height and weight (mg/m2).
Subclass 1: Nitrogen Mustards
In use today are five alkylating agents that are classified as nitrogen mustards. These include mechlorethamine, melphalan, chlorambucil, ifosfamide, and cyclophosphamide. Cyclophosphamide is the most widely used nitrogen mustard and can be found in the regimens for many different cancers. Cyclophosphamide and ifosfamide pose the risk of hemorrhagic cystitis, usually when high-dose therapy is used. To prevent this, a rescue agent, or uroprotectant, is given to prevent bladder irritation. Mesna is the rescue agent used with these drugs for supportive interventions to prevent bladder complications.
Subclass 2: Nitrosoureas
Nitrosoureas are classified as alkylating agents, yet these have an advantage that nitrogen mustards do not: they cross the blood–brain barrier. This characteristic allows these drugs to pass into the brain, treating cancers like glioblastomas and other brain tumors. Carmustine is a more commonly used intravenous nitrosourea. Lomustine, which is similar to carmustine, is administered via oral routes.
Subclass 3: Alkyl Sulfonates
Alkyl sulfonates such as busulfan are administered as an intravenous (IV) infusion rather than IV push. These are very potent drugs, used to treat chronic leukemia, and they have severe adverse effects. These include myelosuppression, stomatitis, nausea, vomiting, diarrhea, and electrolyte imbalances including hypomagnesemia and hypokalemia. Busulfan requires very close pharmacokinetic monitoring and dose alteration.
Chemotherapy, while it acts to target cell functions and reproduction, may cause widespread undesired damage to normal cells. For this reason, many chemotherapeutic agents have black box warnings. These warnings are used to emphasize the most serious and potentially damaging side effects of a drug or a class of drugs. Boxed warnings are set specifically to make clients and health care providers aware of very serious risks of using certain medications. With chemotherapeutics, there are multiple reasons that a boxed warning may be issued. However, the most common reasons include myelosuppression, teratogenicity, and hypersensitivity reactions. Prior to administering a chemotherapeutic drug, nurses should check for boxed warnings so that drugs may be administered safely and clients may be educated regarding these warnings.
Table 8.3 lists common alkylating agents and typical routes and dosing for adult clients.
| Drug | Routes and Dosage Ranges |
|---|---|
| Nitrogen Mustards | |
| Cyclophosphamide (Cytoxan) |
Oral: 1–5 mg/kg once daily. Intravenous (IV) (monotherapy): 40–50 mg/kg given in divided doses over 2–3 days, or 10–15 mg/kg given every 7–10 days. IV (combination therapy): 3–5 mg/kg given twice weekly. Note: Doses may be reported in grams versus milligrams. |
| Chlorambucil (Lukeran) |
0.1–0.2 mg/kg orally once daily for 3–6 weeks. |
| Nitrosoureas | |
| Carmustine (BCNU) |
150–200 mg/m2 IV every 6 weeks administered as a single dose or divided injections on 2 successive days. |
| Lomustine (CCNU) |
130 mg/m2 orally every 6 weeks. |
| Alkyl Sulfonates | |
| Busulfan (Busulfex) |
Clients >12 kg: 0.8 mg/kg IV as a single treatment, every 6 hours for 16 doses. |
| Alkylating-Like Drugs | |
| Cisplatin | Advanced testicular cancer: 20 mg/m2 IV daily for 5 days. Advanced ovarian cancer: 75–100 mg/m2 IV once every 3–4 weeks. Advanced bladder cancer: 50–70 mg/m2 IV once every 3–4 weeks. |
| Carboplatin (Paraplatin) |
300 mg/m2 IV (combination therapy) or 360 mg/m2 (monotherapy) every 4 weeks. |
| Oxaliplatin (Eloxatin) |
85 mg/m2 IV every 2 weeks. |
Adverse Effects and Contraindications
Alkylating agents are some of the most toxic chemotherapies used in cancer treatment. One benefit of these toxicities is that drugs like busulfan can be used to eradicate bone-marrow production prior to stem-cell and bone-marrow transplants. This reduces recurrence of the primary disease after transplant. However, alkylating agents are known for the negative effects associated with administration of these drugs. Short-term side effects include myelosuppression, which usually occurs 6–10 days after administration, with recovery after 14–21 days. Myelosuppression resulting in neutropenia can result in severe infections and sepsis. Mucositis, nausea, and vomiting are side effects that affect the client’s overall nutritional status. Neurotoxicity and alopecia are also short-term side effects. Long-term (delayed) effects of alkylating agents include pulmonary fibrosis, infertility, and secondary malignancies (Amjad & Kasi, 2020). These long-term effects may occur months after treatment or may develop many years later.
Table 8.4 is a drug prototype table for alkylating agents featuring cyclophosphamide. It lists drug class, mechanism of action, adult dosage, indications, therapeutic effects, drug and food interactions, adverse effects, and contraindications.
| Drug Class Alkylating agent–nitrogen mustard Cell cycle nonspecific
Mechanism of Action |
Drug Dosage Oral: 1–5 mg/kg once daily. Intravenous (IV) (monotherapy): 40–50 mg/kg given in divided doses over 2–3 days, or 10–15 mg/kg given every 7–10 days. IV (combination therapy): 3–5 mg/kg given twice weekly. Note: Doses may be reported in grams versus milligrams. |
| Indications Lymphomas Multiple myeloma Leukemia Ovarian and breast cancers
Therapeutic Effects |
Drug Interactions Protease inhibitors Angiotensin-converting enzyme (ACE) inhibitors Thiazide diuretics Zidovudine Amiodarone |
| Adverse Effects Myelosuppression Sepsis Nephrotoxicity Pulmonary toxicity Cardiotoxicity Infertility Hyponatremia |
Contraindications Myelosuppression Urinary outflow obstructionCaution: Monitor for hemorrhagic cystitis Mesna may be used in high-dose therapy to prevent bladder irritation |
Nursing Implications
The nurse should do the following for clients who are taking alkylating agents:
- Assess client overall well-being prior to chemotherapy administration, including vital signs, hydration status, and weight.
- Review laboratory values thoroughly, including complete blood counts, electrolyte profiles, serum creatinine, and liver enzymes.
- Observe clients for adverse effects before, during, and after treatment.
- Ensure patency of intravenous access sites and monitor these frequently during drug administration.
- Adhere to proper handling and administration procedures when administering chemotherapies.
- Be prepared to manage extravasation and follow spill protocols.
- Become familiar with the drug’s black box warnings.
- Recognize and manage emergent situations such as hypersensitivity reactions, bleeding, and sepsis.
- Assess for and provide supportive therapies as needed.
- Provide for educational, spiritual, and psychosocial needs of the client and caregivers.
- Provide client teaching regarding the drug and when to call the health care provider. See below for client teaching guidelines.
Client Teaching Guidelines
The client taking an alkylating drug should:
- Report the following signs and symptoms to the health care provider: fever, chills, productive cough, urinary symptoms, hematuria (cyclophosphamide), changes in hearing (cisplatin).
- Remain well hydrated. Report side effects including nausea and vomiting and mucositis.
- Know how to care for long-term intravenous access at home.
- Understand the need for frequent follow-up and laboratory tests.
- Know which drug/food interactions to avoid.
- Monitor for and report any long-term effects of the chemotherapy.
- Follow up with all recommended health screenings.
- Report any concerning signs and symptoms to their health care provider.
The client taking an alkylating drug should not:
- Be around others who are ill or who have received live vaccines within 3 months.
- Garden without the use of gloves to protect their hands from the risk of bacterial contamination.
- Clean feline litter boxes to decrease the risk of exposure to bacteria or parasites.
- Take vaccines without their health care provider’s approval.
- Consume uncooked meats and wild game such as deer, rabbits, and pheasants.
- Begin taking new supplements or medications without consulting their health care provider.
- Become pregnant.
Various Alkylating Agents
Busulfan injection causes severe and prolonged myelosuppression at the recommended dosage.
Carboplatin causes severe bone marrow suppression, resulting in bleeding, infection, and anemia. Anaphylactic-like reactions to carboplatin may occur within minutes of administration.
Carmustine causes bone marrow suppression, which may contribute to bleeding and overwhelming infection. In cumulative doses above 1400 mg/m2, pulmonary toxicity is a significant risk.
Chlorambucil causes severe bone marrow suppression and infertility and is carcinogenic, mutagenic, and teratogenic.
Cisplatin causes severe renal toxicity that is dose related and cumulative. Peripheral neuropathy occurs and is cumulative with repeat courses. Cisplatin causes severe nausea and vomiting. Bone marrow suppression may be severe, requiring interruption of therapy.
Lomustine causes severe, dose-related, delayed, and cumulative myelosuppression, occurring 4–6 weeks after administration. Overdose is fatal. Only 1 dose should be dispensed with each prescription.
Oxaliplatin may cause anaphylactic-like reactions that may occur within minutes of administration.
Antimetabolites
Antimetabolite chemotherapies are a group of drugs that prevent cancer cell growth by imitating metabolites, which are substances necessary for tumor cell growth. Cancer cells use these substances, which, once inside the cell, prevent DNA replication. This results in cell death. Within this class, there are three types of metabolites that are inhibited: purines, pyrimidines, and folic acid. Each drug in this class specifically targets replacement of one of these substances. The most common drugs within this class are fluorouracil, fludarabine, gemcitabine, and methotrexate. Common side effects include nausea and vomiting, diarrhea, anorexia, stomatitis, alopecia, and myelosuppression. Antimetabolites are useful in treating leukemias, lymphomas, and cancers of the gastrointestinal and biliary tracts.
Table 8.5 lists common antimetabolite agents and typical routes and dosing for adult clients.
| Drug | Routes and Dosage Ranges |
|---|---|
| Folate Antimetabolites | |
| Methotrexate (Trexall) |
Intrathecal: 6–15 mg/m2 age-based dose; frequency depends on regimen. IV: 10 mg/m2 up to 12,000 mg/m2 at varying frequencies depending on the diagnosis and treatment regimen. |
| Pemetrexed (Alimta) |
500 mg/m2 IV every 21 days. |
| Pyrimidine Antimetabolites | |
| 5-Fluorouracil (5-FU) |
Colon/rectal adenocarcinoma: 400 mg/m2 IV bolus followed by 2400–3000 mg/m2 continuous infusion over 46 hours every 2 weeks. Pancreatic adenocarcinoma: 400 mg/m2 IV bolus followed by 2400 mg/m2 continuous infusion over 46 hours every 2 weeks. Breast adenocarcinoma: 500–600 mg/m2 IV days 1 and 8 every 28 days for 6 cycles. Gastric adenocarcinoma: 200–1000 mg/m2 IV over 24 hours at varying frequencies depending on regimen. |
| Capecitabine (Xeloda) |
1250 mg/m2 orally twice daily for 2 weeks followed by a 1-week rest period. |
| Cytarabine (Ara-C) |
IV: 100 mg/m2 daily as a single treatment over 7 days. Intrathecal: 5–75 mg/m2 once every 4 days. |
| Purine Antimetabolites | |
| Mercaptopurine (Purixan) |
1.5–2.5 mg/kg orally once daily. |
| Thioguanine (Tabloid) |
2–3 mg/kg orally daily. |
| Gemcitabine (Gemzar) |
1000 mg/m2 IV on days 1 and 8 of a 21-day cycle. |
| Fludarabine (Fludara) |
25 mg/m2 IV daily for 5 days, every 28 days. |
Adverse Effects and Contraindications
Antimetabolites are associated with many adverse effects. Folate antimetabolites are associated with myelosuppression, mucositis, hepatotoxicity, nephrotoxicity, and cutaneous reactions. Pyrimidine antimetabolites cause mucositis and myelosuppression as well but are also associated with dose-limiting hand-foot syndrome and diarrhea. Contraindications for fluorouracil include clients with dihydropyridine dehydrogenase (DPD) deficiency, which may result in toxic levels of fluorouracil. This can lead to cardiac dysfunction, colitis, neutropenia, and encephalopathy. Uridine triacetate is used to treat toxicity in these clients. Cytarabine is associated with inflammation of the conjunctiva. Corticosteroid eye drops are used prophylactically to prevent this. Purine antimetabolites, in addition to causing myelosuppression, also decrease the CD4 lymphocyte count, resulting in immunosuppression and risk of opportunistic infections (Amjad & Kasi, 2020).
Table 8.6 is a drug prototype table for antimetabolites featuring fluorouracil. It lists drug class, mechanism of action, adult dosage, indications, therapeutic effects, drug and food interactions, adverse effects, and contraindications.
| Drug Class Antimetabolite (pyrimidine antagonist)
Mechanism of Action |
Drug Dosage Colon/rectal adenocarcinoma: 400 mg/m2 IV bolus followed by 2400–3000 mg/m2 continuous infusion over 46 hours every 2 weeks. Pancreatic adenocarcinoma: 400 mg/m2 IV bolus followed by 2400 mg/m2 continuous infusion over 46 hours every 2 weeks. Breast adenocarcinoma: 500–600 mg/m2 IV days 1 and 8 every 28 days for 6 cycles. Gastric adenocarcinoma: 200–1000 mg/m2 IV over 24 hours at varying frequencies depending on regimen. |
| Indications Breast, colon, pancreatic, and gastric cancers
Therapeutic Effects |
Drug Interactions Warfarin |
| Adverse Effects Mucositis Diarrhea Hand-foot syndrome Myelosuppression Neurotoxicity Gastrointestinal ulcers |
Contraindications Hypersensitivity Decreased dipyridine dehydrogenase
Caution: |
Nursing Implications
The nurse should do the following for clients who are taking antimetabolite agents:
- Assess client overall well-being prior to chemotherapy administration, including vital signs, hydration status, oral mucosa, skin, eyes (cytarabine), and weight.
- Review laboratory values thoroughly, including complete blood counts, electrolyte profiles, serum creatinine, and liver enzymes. Observe clients for adverse effects before, during, and after treatment.
- Ensure patency of intravenous access sites and monitor these frequently during drug administration.
- Adhere to proper handling and administration procedures when administering chemotherapies.
- Be prepared to manage extravasation and spill protocols.
- Be aware of drugs’ boxed warnings.
- Recognize and manage emergent situations such as hypersensitivity reactions, bleeding, and sepsis.
- Assess for and provide supportive therapies as needed.
- Provide for educational, spiritual, and psychosocial needs of the client and caregivers.
- Provide client teaching regarding the drug and when to call the health care provider. See below for client teaching guidelines.
Client Teaching Guidelines
The client taking an antimetabolite agent should:
- Report the following signs and symptoms to the health care provider: fever, chills, productive cough, urinary symptoms, hematuria, eye irritation (cytarabine), and mouth ulcers (fluorouracil).
- Remain well hydrated.
- Report side effects including nausea and vomiting and mucositis.
- Know how to care for long-term intravenous accesses at home.
- Understand the need for frequent follow-up and laboratory tests.
- Know which drug/food interactions to avoid.
- Monitor and report any long-term effects.
- Follow up with recommended screenings for early identification of any secondary malignancies.
The client taking an antimetabolite should not:
- Be around others who are ill or who have received live vaccines within 3 months.
- Garden without the use of gloves to decrease the risk of exposure to mold and bacteria.
- Clean feline litter boxes to minimize bacteria or parasite exposure.
- Take vaccines without consulting with their health care provider.
- Consume uncooked meats and wild game such as deer, rabbits, and pheasants.
- Begin taking new supplements or medications without consulting their health care provider.
- Become pregnant.
FDA Black Box Warning
Antimetabolites
Methotrexate causes embryo-fetal toxicity, hypersensitivity reactions, benzyl alcohol toxicity, and other serious adverse reactions.
Capecitabine causes increased risk for bleeding when administered with coumarins such as warfarin.
Fludarabine causes severe central nervous system toxicity, including blindness, coma, seizures, and death. Autoimmune syndromes including hemolytic anemia, thrombocytopenia, and hemophilia may occur with fludarabine administration. Concomitant use of deoxycoformycin (pentostatin) may cause fatal pulmonary toxicity.
Anthracyclines/Antitumor Antibiotics
Anthracyclines are some of the most potent chemotherapies on the market today. These drugs are very strong vesicants that cause severe necrosis when extravasated. Three major drugs in this class are daunorubicin, doxorubicin, and epirubicin. These drugs cause cell death by preventing DNA replication. They do this by inhibiting topoisomerase, leaving DNA strands unable to unwind and replicate. The major side effects of anthracyclines are myelosuppression, nausea and vomiting, alopecia, skin and nail hyperpigmentation, and, most notably, cardiotoxicity. Many drugs in this class are red in color and can resultingly cause urine and other body fluids to turn red/orange. This is a benign side effect but is usually discussed with clients to avoid any panic. These drugs are assigned maximum lifetime dose limits to aid in preventing debilitating chemotherapy-induced heart failure. Additionally, clients must have a left ventricular ejection fraction of at least 55% to receive these drugs. Table 8.7 lists common anthracyclines/antitumor antibiotics and typical routes and dosing for adult clients.
| Drug | Drug Routes and Dosages |
|---|---|
| Anthracyclines/Antitumor Antibiotics | |
| Daunorubicin (Cerubidine) |
25–45 mg/m2 IV; frequency depends on cancer type and other agents administered in combination (550 mg/m2 maximum lifetime limit due to cardiac toxicity). |
| Doxorubicin (Adriamycin, Doxil) |
60–75 mg/m2 IV every 21 days (550 mg/m2 maximum lifetime limit due to cardiac toxicity). |
| Epirubicin (Ellence) |
IV: 100–120 mg/m2 frequency depends on prescribed regimen (720 mg/m2 maximum lifetime limit due to cardiac toxicity). |
| Other Anthracyclines | |
| Bleomycin (Blenoxane) |
0.25–0.5 units/kg (10–20 units/m2) given IV, intamuscularly, or subcutaneously weekly or twice weekly. (Drug may be discontinued if pulmonary toxicity occurs.) |
| Dactinomycin (Cosmegen) |
12–1250 mcg/m2 IV; frequency depends on cancer type. |
| Mitomycin (Mutamycin) |
20 mg/m2 IV at 6–8 week intervals. |
Adverse Effects and Contraindications
Anthracyclines and antitumor antibiotics are associated with very serious adverse effects. In general, the drugs cause significant myelosuppression, alopecia, and nausea and vomiting. Doxorubicin and daunorubicin are associated with both short- and long-term cardiotoxicity. These drugs are contraindicated in clients with preexisting cardiac disease when the left ventricular ejection fraction is less than 55%. Clients receiving doxorubicin are also limited to a lifetime cumulative dose of 550 mg/m2. Bleomycin administration requires vigilant monitoring for cumulative pulmonary toxicity and fibrosis. Doxorubicin and daunorubicin are vesicant drugs that cause severe necrosis if extravasation into tissues occurs.
Table 8.8 is a drug prototype table for anthracycline agents featuring doxorubicin. It lists drug class, mechanism of action, adult dosage, indications, therapeutic effects, drug and food interactions, adverse effects, and contraindications.
| Drug Class Anthracycline/antitumor antibiotic
Mechanism of Action |
Drug Dosage 60–75 mg/m2 IV every 21 days (550 mg/m2 maximum lifetime limit due to cardiac toxicity). |
| Indications Breast, bronchogenic, and thyroid cancers Leukemia Lymphoma Sarcoma Wilms tumor
Therapeutic Effects |
Drug Interactions Paclitaxel Trastuzumab 6-mercaptopurine Dexrazoxane (may be used as a cardioprotective agent in certain populations or upon extravasation) |
| Adverse Effects Cardiotoxicity Myelosuppression Alopecia Hyperpigmentation of skin and nails Stomatitis |
Contraindications Hypersensitivity Myelosuppression Decreased cardiac function
Caution: |
Nursing Implications
The nurse should do the following for clients who are taking anthracyclines/antitumor antibiotic agents:
- Assess client overall well-being prior to chemotherapy administration including vital signs, hydration status, oral mucosa, skin, and weight.
- Review laboratory values thoroughly, including complete blood counts, electrolyte profiles, serum creatinine, liver enzymes, and left ventricular ejection fraction and pulmonary function tests (bleomycin).
- Carefully record cumulative doses when lifetime limits are necessary.
- Observe clients for adverse effects before, during, and after treatment.
- Ensure patency of intravenous access sites and monitor these frequently during drug administration.
- Adhere to proper handling and administration procedures when administering chemotherapies.
- Be prepared to manage extravasation and follow spill protocols.
- Be aware of the drug’s black box warnings.
- Recognize and manage emergent situations such as hypersensitivity reactions, bleeding, and sepsis.
- Assess for and provide supportive therapies as needed.
- Provide for educational, spiritual, and psychosocial needs of the client and caregivers.
- Provide client teaching regarding the drug and when to call the health care provider. See below for client teaching guidelines.
Client Teaching Guidelines
The client taking an anthracycline/antitumor antibiotic agent should:
- Know signs and symptoms to report to the health care provider, including fever, chills, productive cough, urinary symptoms, hematuria, palpitations, chest pain, shortness of breath (doxorubicin, daunorubicin, epirubicin), pulmonary pain, and breathing difficulties (bleomycin).
- Remain well-hydrated.
- Report side effects including nausea and vomiting and mucositis.
- Know how to care for long-term intravenous accesses at home.
- Understand the need for frequent follow-up and laboratory tests.
- Know which drug/food interactions to avoid.
- Follow up with screenings for long-term effects and secondary malignancies.
The client taking an anthracycline and antitumor antibiotic agent should not:
- Be around others who are ill or who have received live vaccines within 3 months.
- Garden without the use of gloves to protect hands from direct contact with bacteria and mold present in the soil.
- Clean feline litter boxes to reduce risk of contact with bacteria and parasites.
- Take vaccines without prior approval of their health care provider.
- Consume uncooked meats and wild game such as deer, rabbits, and pheasants.
- Begin taking new supplements or medications without consulting their health care provider.
- Become pregnant.
FDA Black Box Warning
Doxorubicin and Epirubicin
Potentially fatal congestive heart failure can occur during or years after therapy. The probability is based on the total cumulative doses received.
Secondary acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) has been reported in clients taking these medications.
Plant Alkaloids
Several chemotherapies are derived from plants and are considered plant alkaloids. Vinca alkaloids are the most common plant alkaloids. Vincristine, vinblastine, and etoposide (VP-16) are all plant alkaloids that cause misalignment of chromosomes in cancer cells, resulting in apoptosis, or cell death. These are useful in treating lymphomas, leukemias, Kaposi sarcoma, squamous cell carcinomas, lung cancer, and bladder cancer. Side effects include myelosuppression, mouth sores, nausea, vomiting, and fatigue. The most significant adverse effects involve the nervous system. Because plant alkaloids decrease nerve function, these substances can cause hearing loss, neuropathies, and severe constipation that may develop into a paralytic ileus. Neurologic symptoms may necessitate discontinuance of therapy. Vinca alkaloids are often part of regimens that also require intrathecal administration. However, close attention and safety measures (i.e., drug is always compounded in an IV bag for infusion) must be in place to ensure these are never administered via the intrathecal route. The drugs are fatal if administered intrathecally.
Table 8.9 lists common plant alkaloids and typical routes and dosing for adult clients.
| Drug | Routes and Dosage Ranges |
|---|---|
| Vincristine (Oncovin) |
1.4 mg/m2 IV weekly. |
| Vinblastine (Velban) |
3.7 mg/m2 IV initial dose; subsequent doses up to 18.5 mg/m2 administered weekly. |
| Etoposide (Vepesid, VP-16) |
35–100 mg/m2 IV; frequency depends on cancer type and other agents administered. |
Adverse Effects and Contraindications
Peripheral neuropathy is a common but serious adverse effect of plant alkaloids. Both motor and sensory functions are affected, and neuropathy can be severe enough to result in paralytic ileus. Myelosuppression is another adverse effect caused by plant alkaloids. These drugs are classified as irritants, which may cause significant irritation to the skin should extravasation occur.
Table 8.10 is a drug prototype table for plant alkaloids featuring vincristine. It lists drug class, mechanism of action, adult dosage, indications, therapeutic effects, drug and food interactions, adverse effects, and contraindications.
| Drug Class Plant alkaloidMechanism of Action Causes chromosomal link errors, results in apoptosis of cancerous cells |
Drug Dosage 1.4 mg/m2 IV weekly. |
| Indications Lymphoma Leukemia Kaposi sarcoma Squamous cell carcinoma Lung cancer Bladder cancer
Therapeutic Effects |
Drug Interactions Anticonvulsants Amiodarone Carvedilol Erythromycin Fluconazole Rifampin Warfarin
Food Interactions |
| Adverse Effects Neuropathy Severe constipation Paralytic ileus Urinary retention Hearing loss Alopecia |
Contraindications Hypersensitivity Charcot-Marie-Tooth disease
Caution: |
Nursing Implications
The nurse should do the following for clients who are taking plant alkaloid agents:
- Assess client overall well-being prior to chemotherapy administration including vital signs, hydration status, oral mucosa, skin, weight, bowel function, and signs of neuropathy.
- Review laboratory values thoroughly, including complete blood counts, electrolyte profiles, serum creatinine, and liver enzymes.
- Observe clients for adverse effects before, during, and after treatment.
- Inspect drug preparation to ensure that these drugs are properly mixed and prepared as intravenous infusions. Do not administer these medications directly to the client using a syringe. This will decrease the risk of having the medication erroneously administered intrathecally, which can be fatal. Ensure patency of intravenous access sites and monitor these frequently during drug administration.
- Adhere to proper handling and administration procedures when administering chemotherapies.
- Be prepared to manage extravasation and follow spill protocols.
- Be aware of the drug’s black box warnings.
- Recognize and manage emergent situations such as hypersensitivity reactions, bleeding, and sepsis.
- Assess for and provide supportive therapies as needed.
- Provide for educational, spiritual, and psychosocial needs of the client and caregivers.
- Provide client teaching regarding the drug and when to call the health care provider. See below for client teaching guidelines.
Client Teaching Guidelines
The client taking a plant alkaloid agent should:
- Report the following signs and symptoms to the health care provider: fever, chills, productive cough, urinary symptoms, hematuria, decreased bowel elimination or constipation, hearing loss, and peripheral neuropathy.
- Remain well hydrated and use stool softeners and laxatives when needed.
- Report side effects including nausea and vomiting, mucositis, and constipation.
- Learn how to care for long-term intravenous accesses at home.
- Understand the need for frequent follow-up and laboratory tests.
- Know which drug/food interactions to avoid.
- Understand the need for surveillance for long-term effects and secondary malignancies.
The client taking a plant alkaloid agent should not:
- Be around others who are ill or who have received live vaccines within 3 months.
- Garden without the use of gloves to avoid direct contact with potentially contaminated soil.
- Clean feline litter boxes to protect hands from contamination by bacteria or parasites.
- Take vaccines without consulting with their health care provider.
- Consume uncooked meats and wild game such as deer, rabbits, and pheasants.
- Begin taking new supplements or medications without consulting their health care provider.
- Become pregnant.
Taxanes
Taxanes are a group of chemotherapeutic agents that were developed from the bark of a yew tree. These are effective in the treatment of breast, ovarian, prostate, gastric, esophageal, pancreatic, and non-small cell lung cancers as well as Kaposi sarcoma. These agents are typically used in combination with other agents rather than as a monotherapy. Adverse reactions include, most notably, hypersensitivity reactions. For this reason, clients will be premedicated, usually with corticosteroids, but may also receive diphenhydramine and a histamine-2 receptor antagonist to prevent these reactions from occurring. Other adverse effects include hepatotoxicity, fluid retention, myelosuppression, alopecia, skin and nail changes, and nausea, vomiting, and diarrhea.
Table 8.11 lists common taxanes and typical routes and dosing for adult clients.
| Drug | Routes and Dosage Ranges |
|---|---|
| Docetaxel (Taxotere) |
60–100 mg/m2 IV every 3 weeks. |
| Paclitaxel (Taxol) |
100–175 mg/m2 IV every 3 weeks. |
Adverse Effects and Contraindications
Taxanes are most known for the adverse effect of hypersensitivity reactions. A test dose and premedication with antihistamines and acetaminophen may be used prior administration. Myelosuppression may result in lowered levels of platelets and white blood cells. Taxanes are contraindicated in solid tumors with myelosuppression with neutrophil counts under 1500 cells/mm3. Peripheral neuropathy is also commonly associated with taxanes, resulting in pain and limited movement. Fatigue and arthralgias are also seen with these drugs. Cardiovascular changes including hypotension, bradycardia, hypertension, and electrocardiogram (ECG, EKG) changes may be noted (DailyMed, Paclitaxel, 2023).
Table 8.12 is a drug prototype table for taxanes featuring paclitaxel. It lists drug class, mechanism of action, adult dosage, indications, therapeutic effects, drug and food interactions, adverse effects, and contraindications.
| Drug Class Taxane
Mechanism of Action |
Drug Dosage 100–175 mg/m2 IV every 3 weeks. |
| Indications Breast, ovarian, and non-small cell lung cancers Kaposi sarcoma
Therapeutic Effects |
Drug Interactions Midazolam Buspirone Statins Felodipine Protease inhibitors Repaglinide Rifampin |
| Adverse Effects Hypersensitivity reactions Myelosuppression ECG changes Peripheral neuropathy Arthralgia Nausea Vomiting Diarrhea Mucositis Alopecia Infusion site reactions |
Contraindications Hypersensitivity Myelosuppression with neutrophil counts under 1500 µLCaution: May cause anaphylaxis due to the medication vehicle, not the drug itself Must premedicate |
Nursing Implications
The nurse should do the following for clients who are taking taxane agents:
- Assess client overall well-being prior to chemotherapy administration, including vital signs, hydration status, oral mucosa, skin, weight, cardiac function, and signs of neuropathy.
- Review laboratory values thoroughly, including complete blood counts, electrolyte profiles, serum creatinine, and liver enzymes.
- Observe clients for adverse effects before, during, and after treatment.
- Ensure patency of intravenous access sites and monitor these frequently during drug administration.
- Adhere to proper handling and administration procedures when administering chemotherapies.
- Be prepared to manage extravasation and follow spill protocols.
- Be aware of the drug’s black box warnings.
- Recognize and manage emergent situations such as hypersensitivity reactions, bleeding, and sepsis.
- Assess for and provide supportive therapies as needed.
- Provide for educational, spiritual, and psychosocial needs of the client and caregivers.
- Provide client teaching regarding the drug and when to call the health care provider. See below for client teaching guidelines.
Client Teaching Guidelines
The client taking a taxane agent should:
- Recognize signs and symptoms to report to the health care provider: fever, chills, productive cough, urinary symptoms, hematuria, decreased bowel elimination or constipation, hearing loss, and peripheral neuropathy.
- Understand the importance of remaining well hydrated and use stool softeners and laxatives when needed.
- Learn how to manage side effects including nausea and vomiting, mucositis, and constipation.
- Understand how to care for long-term intravenous accesses at home.
- Understand the need for frequent follow-up and laboratory tests.
- Know which drug/food interactions to avoid.
- Understand the need for surveillance and follow-up for management of long-term effects and early identification of secondary malignancies.
The client taking a taxane agent should not:
- Be around others who are ill or who have received live vaccines within 3 months.
- Garden without the use of gloves to protect hands from exposure to bacteria and mold.
- Clean feline litter boxes to protect hands from direct contact with bacteria.
- Take vaccines without consulting with their health care provider.
- Consume uncooked meats and wild game such as deer, rabbits, and pheasants.
- Begin taking new supplements or medications without consulting with their health care provider.
- Become pregnant.
Docetaxel
Docetaxel has been associated with fatalities among clients with abnormal liver function, who are receiving higher doses, with non-small cell carcinoma, or with a history of receiving platinum-based chemotherapy including cisplatin, carboplatin, and oxaliplatin.
Common Drugs Used as Supportive Therapies for Clients Receiving Chemotherapy
Because chemotherapy regimens are so complex, supportive care with other pharmacologic agents is usually required. Management of adverse effects is critical to safe and successful chemotherapy treatment. Myelosuppression and nausea and vomiting are most commonly managed with supportive therapies. Corticosteroids and biologic colony stimulating factors are frontline supportive therapies. Table 8.13 encompasses the most common supportive therapies that may be necessary when administering chemotherapy.
| Classification/Drug | Routes and Dosages | Use |
|---|---|---|
| Corticosteroids | ||
| Dexamethasone (Decadron) |
Oral: 0.75–9 mg/day. IV/intramuscular: 0.5–9 mg/day. |
Decreases nausea and vomiting. Used as premedication to reduce hypersensitivity reactions. |
| Antihistamines | ||
| Diphenhydramine (Benadryl) |
Oral: 25–50 mg every 4–6 hours as needed. IV/intramuscular: 10–50 mg up to 100 mg if required; 400 mg maximum daily dose. |
Treats/prevents hypersensitivity reactions. |
| Loratadine (Allegra) |
Oral: 10 mg 1 hour prior to chemotherapy initiation. | Prevents hypersensitivity reactions. |
| Colony Stimulating Factors | ||
| Filgrastim (Neupogen) |
5–10 mcg/kg/day administered as a single daily subcutaneous or IV injection or by continuous subcutaneous or IV infusion. | Stimulates bone marrow stem cells to produce increased neutrophil production. Prevents infection. |
| Pegfilgrastim (Neulasta) |
6 mg/dose subcutaneously. | Stimulates bone marrow stem cells to produce increased neutrophil production. Prevents infection. |
| Epoetin alfa (Epogen) |
150 units/kg subcutaneously 3 times weekly or 40,000 units/dose weekly; titrated based on hemoglobin response. | Stimulates bone marrow stem cells to produce increased erythocyte production. Prevents/treats anemia. |
These supportive therapies can increase the client’s quality of life as well as decrease risks associated with pancytopenia, such as sepsis and decreased oxygen-carrying capacity of the blood. The advent of biologic colony stimulating factors marked a significant decrease in sepsis-related deaths for clients receiving chemotherapy. Ondansetron, specifically, has greatly changed the way chemotherapy-related nausea and vomiting are treated. Traditional phenothiazines cause vein and tissue irritation as well as central nervous system depression, placing a client at risk for injury. Ondansetron does not affect central nervous system function, reducing the risk of falls and other injuries.
FDA Black Box Warning
Erythropoiesis-Stimulating Agents (ESAs)
Erythropoiesis-stimulating agents (ESAs) increase the risk of death, myocardial infarction, stroke, venous thromboembolism, and tumor progression or recurrence.
Next- 8.5.3 Hormonal Therapy
Access for free at https://openstax.org/books/pharmacology/pages/1-introduction
by OpenStax is licensed under Creative Commons Attribution License v4.
During or relating to the eating of food.
A blood glucose level below 70 mg/dL; severe hypoglycemia refers to a blood glucose level below 40.
Abnormally low blood levels of thyroid hormones T3 and T4 in the bloodstream.
Connection between two neurons, or between a neuron and its target, where a neurotransmitter diffuses across a very short distance.
Chemical signals sent by the endocrine organs and transported via the bloodstream throughout the body where they bind to receptors on target cells and induce a characteristic response.
Long-acting (insulin glargine or insulin detemir) or intermediate-acting (NPH) insulin.
Long-acting (insulin glargine or insulin detemir) or intermediate-acting (NPH) insulin.

